All published articles of this journal are available on ScienceDirect.
Traditional and Advanced Neuroimaging Contributions to the Diagnosis and Differential Diagnosis of Central Nervous System Lymphoma Patients Visiting a Comprehensive Medical Center
Abstract
Introduction:
The diagnosis of Central Nervous System Lymphoma, especially the Primary Central Nervous System Lymphoma is carried out based on brain imaging, thus avoiding an unnecessary extend of surgery. But the traditional imaging techniques, such as Computed Tomography and Magnetic Resonance Imaging, were not satisfactory.
Aims:
This study was conducted to characterize the spectrum of advanced Neuroimaging, such as the advanced Magnetic Resonance Imaging features in the Central Nervous System Lymphoma patients in a comprehensive medical center in Lebanon, and compare them to what has been described in the literature review.
Methods:
It is a retrospective exploratory study of the clinical data and imaging features for patients admitted to the emergency and radiology departments with ages above 10 years, and who were diagnosed histopathologically with intracranial lymphoma. This study may be the first to make a Radiological evaluation of Central Nervous System Lymphoma on the local population of patients over 9 years .
Results:
Results showed that the study of the Computed Tomography and Magnetic Resonance Imaging data of 10 immunocompetent patients with Central Nervous System Lymphoma concurs with the previously described patient populations, except for the gender parameter. Tumors were mostly presented in the fifth or Sixth decade and they could be solitary or multi-focal. Lesions were typically located Preprint submitted to The Open Neuroimaging Journal May 14, 2020 in the supratentorial compartment. On the brain Computed Tomography, the lesions were hyperdense, and in pre-contrast Magnetic Resonance images, the lesions appeared hypointense on T1 and hyperintense on T2-Weighted images, but hypointense with respect to the grey matter. The lesions were also surrounded with a mild to moderate edema as compared to other intracranial neoplasms, such as glioblastomas. Evaluation results showed that on post-contrast Magnetic Resonance images, the majority of lesions exhibited a homogeneous enhancement of 50%. Majority of the lesions also showed a less common heterogeneous ring-like enhancement of 40%, and revealed the uncommon absence of enhancement of 10%. Calcifications, hemorrhage, and necrosis were rare findings and were present in only one patient.
Conclusion:
As a future prospect, studying whether the advanced imaging techniques may provide not only non-invasive and morphological characteristics but also non-invasive biological characteristics and thus accurate diagnosis could be considered.
1. INTRODUCTION
Central Nervous System Lymphoma (CNSL) is a form of extranodal, high-grade non-Hodgkin B-cell lymphoma [1]. CNS lymphoma is divided into two categories, Primary Central Nervous System Lymphoma (PCNSL) and Secondary Central Nervous System Lymphoma (SCNSL). PCNSL is a rare subtype of non-Hodgkin B-cell lymphoma that originates in the brain, meninges, nerves of the eye, or spinal cord, without any evidence of a systemic disease [2]. SCNSL is a rare complication of non-Hodgkin lymphoma; it refers to CNS spread of lymphoma that originated elsewhere, outside the CNS [3].
Before the year 1970, PCNSL accounted for less than 1% of brain neoplasms, however, the incidence has greatly increased during the last two decades. It now represents 6.6% of primary intracranial neoplasms [4]. The increasing use of chemotherapy and chemoradiotherapy in the recent years have significantly improved the lives of patients compared to the use of radiotherapy merely [5]. Most symptomatic patients with PCNSL present with large lesions, thus steroids are frequently administered before any surgical procedure, rendering a low diagnostic yield of resection, stereotactic biopsy or Cerebrospinal Fluid (CSF) examination. Therefore, the study of early recognition of PCNSL by medical imaging is essential to avoid steroids and to facilitate attempts to take a biopsy, in a partially invasive manner, rather than resection which is a fully invasive procedure, and does not improve the prognosis [6]. The traditional imaging features have been described and characterized in many previous studies [7-9], but none of the reports have studied the demographic features/parameters, especially in a comprehensive medical center, such as Mount Lebanon Medical Center in Lebanon. Therefore, this study may be the first to explore the features/parameters extracted from Neuroimaging and Radiological approaches to evaluate CNSL on the local population of patients. The aim of this study is also to compare the findings from the population to what has been described in the literature reports and reviews on CNSL.
The remainder of the paper is organized as follows. In section 2, the materials including the imaging machines and specifications are provided. In section 3, the method is reported, including the criteria utilized. In section 4, the results are reported in tables and shown in figures. In section 5, the reported results are discussed. In section 6, the general conclusion of the work is provided. Finally, in section 7, the future work is reported.
2. MATERIALS
An imaging database containing a retrospective review of brain tumors in the pathology archives at Mount Lebanon Medical Center was utilized, from 2009 to 2018. This retrospective study was conducted in the Radiology Department at Mount Lebanon Medical Center and was approved by the Neuroimaging Research Board at the Neuroscience Research Center of the Faculty of Medical Sciences at the Lebanese University.
Brain images were obtained through a 1.5 Tesla Magnetic Resonance Imaging (MRI), Siemens machine. The contrast used in all MRI exams was the Gadolinium. Moreover, brain images were collected through the 20-slices spiral Computed Tomography (CT) Siemens machine. Besides, the results were evaluated using GraphPad Prism Software.
3. METHODS
A retrospective study of brain tumors in the pathology archives of patients visiting the Emergency Department (ED) and the Radiology Department (RD) over 9 years revealed 10 cases of CNS lymphoma. Then medical history and clinical database for the 10 patients were reviewed for the presence of a history of Immunodeficiency (ID). Only patients without a history of acquired ID syndrome or other congenital ID disorders were included in the study. Collected demographic data included age, gender, and lymphoma type. Medication sheet was then reviewed for received medical treatment, including corticosteroids, chemotherapy, and radiotherapy. Only patients who had MRI and CT scan examinations performed before administration of steroids and prior to further treatment were included. All 10 patients were eligible for inclusion.
The imaging data were reviewed for Positron Emission Tomography (PET) scans and cerebral images, including CT scan and MRI. The Gadolinium contrast agent was administered at a dose of 0.16 UI body weight. Two experienced radiologists reviewed and analyzed the images. All scans were reviewed noting lesion location in the brain, size, margins, and the signal characteristics. The presence of calcifications, hemorrhage, perilesionaledema, meningeal enhancement, and the characteristics of enhancement were also examined. All patients had cerebral CT scans with and without contrast, T1, T2, T2*, Fluid Attenuation Inversion Recovery (FLAIR), and the advanced medical imaging, such as the post contrast T1 weighted, and Diffusion-Weighted Images (DWI). The followed methodology comprised the inclusion and exclusion criteria.
3.1. Inclusion and Exclusion Criteria
The inclusion criteria included:
• Patients aged 10 years and above.
• Patients who had biopsy and were diagnosed as CNSL Patients;
• Patients had brain CT and/or brain MRI on presentation.
• Patient had PET scan or CT for the chest, abdomen, and pelvis to distinguish PCNSL from SCNSL.
The exclusion criteria included:
• Patients aged less than 10 years.
• Patients received corticosteroid treatment, underwent radiotherapy, or other treatments before imaging was obtained. Thereby, they were excluded from the statistical evaluation.
3.2. Statistical Evaluation Method
The evaluation of the results was conducted through the statistical methods embedded in GraphPad Prism Software. The Chi-Square test (X2 test) of independence was used to determine if there is a significant relationship between the two extracted categorical features/parameters. All tests were two-sided and a p−value < 0.05 was considered statistically significant.
4. RESULTS
After applying the aforementioned methods and using the reported materials, the results were obtained. The results were divided into both the Clinical findings and the Neuro-Radiological findings and are summarized in Tables 1 and 3, respectively.
4.1. Results of Brain Tumor Type
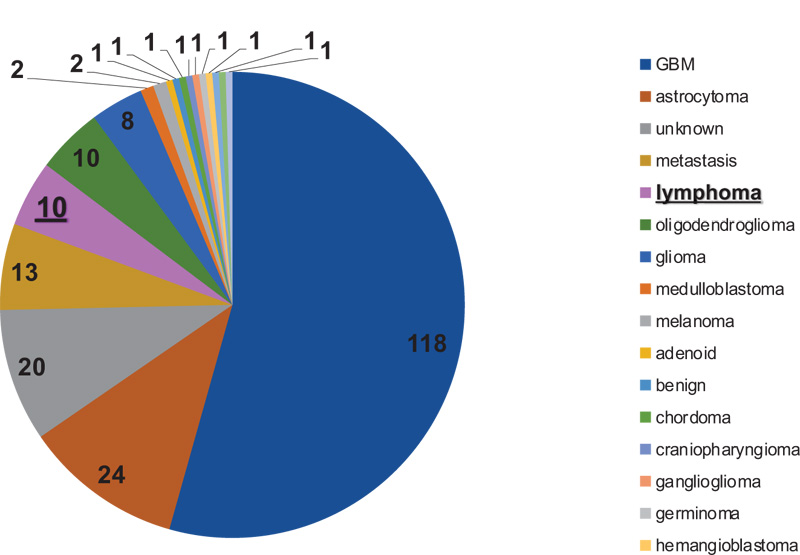
A total of 227 patients, aged above 10 years, have undergone brain biopsy at Mount Lebanon Medical Center. Out of all the explored patients, 10 patients were found to have lymphoma proven pathologically, as shown in Fig. (1) and Table 1. Thereby, out of all patients administrated to ED, 5% of them had lymphoma.
4.2. Results of Age
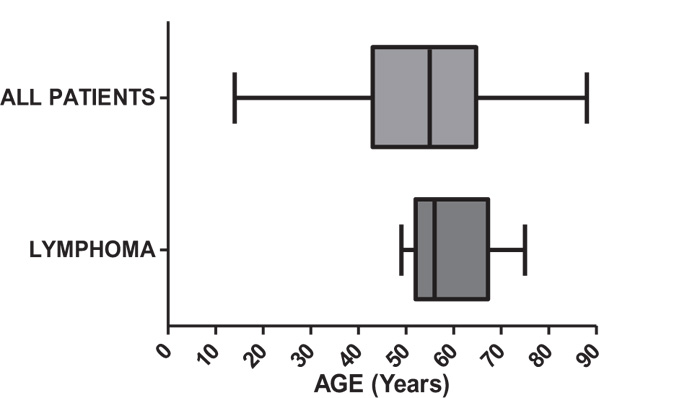
The age distribution, as shown in Fig. (2) and Table 1, revealed that the median age of patients diagnosed with lymphoma was 56 years, with a range from 49 to 75 years. Moreover, the medium age of patients diagnosed with other brain tumors was 55 years, with a wider range from 14 to 88 years.
4.3. Results of Gender
Out of all the explored patients (M=227) with brain tumors, 98 patients were females and 129 were males. 8.1% (N=8) of female patients were diagnosed histopathologically with lymphomas. However, only 1.57% (N=2) of male patients were diagnosed histopathologically to have lymphomas. Concerning lymphoma patients, (N=10) females represented 80% (N=8), while males represented only 20% (N=2), as reported in Table 2.
| Patient | Gender | Age | Presenting Symptoms | Immune Status | Histology |
|---|---|---|---|---|---|
| 1 | Male | 75 | Nausea, Vertigo, Ataxia | IC | DLBL |
| 2 | Female | 56 | Headache, Seizure | IC | DLBL |
| 3 | Female | 63 | Confusion, Hallucinations | IC | DLBL |
| 4 | Female | 62 | Left-Sided Hemiparesis, Insomnia | IC | DLBL |
| 5 | Female | 46 | Left-Sided Numbness, Photophobia | IC | DLBL |
| 6 | Male | 52 | Vertigo, Apahasia, Photophobia | IC | DLBL |
| 7 | Female | 55 | Behavioral Changes | IC | DLBL |
| 8 | Female | 54 | Confusion, Headache | IC | DLBL |
| 9 | Female | 75 | Right-Sided Numbness, Agitation | IC | DLBL |
| 10 | Female | 52 | Headache | IC | DLBL |
| Gender | Patients with CNS Lymphoma (N=10) | Patients with Other Brain Tumor | P-Value |
|---|---|---|---|
| Females | 80% (8) | 41.47% (90) | 0.0162 |
| Males | 20% (2) | 58.53% (127) | 0.0162 |
In order to test the association between gender and the risk of developing lymphoma, the Chi-Square test of independence was used. There was a statistically significant association between female gender and developing lymphoma with a p-value of 0.0162 < 0.05. The females have 5.6 times higher risk to develop lymphoma as compared to males (odd ratio 5.644).
4.4. Results of Type of Lymphoma
The results of lymphoma classification to either PCNSL or SCNSL is illustrated in Fig. (3) and Table 3. The classification was based on the results of both brain biopsy and PET scan or CT for the chest, abdomen, and pelvis with contrast. The resulting distribution of CNSL patients was according to different variables:
• Immune status, i.e. Immunocompetent (IC) versus immunocompromised.
• Location of the lesion on imaging, i.e. infratentorial versus supratentorial.
• Number of lesions, i.e. solitary versus multi-focal.
• Post-contrast enhancement.
| Patient | Type of CNSL | Number of Lesions | Size | Location | Surrounding Edema | Gadolinium Enhancement | Signal intensity | Others | |
|---|---|---|---|---|---|---|---|---|---|
| T1 | T2 | ||||||||
| 1 | PCNSL | 1 | 35 mm | Left cerebellar | Mild | Homogeneous | Hypo | Hyper | Intralesional hemorrhage |
| 2 | SCNSL | 4 | 22 mm* | Supratentorial | Mild | Heterogeneous | Hypo | Hyper | Hemorrhagic Component |
| 3 | PCNSL | 1 | 70 mm | Right temporoparietal | Important | Heterogeneous | Hyper | Hyper | Midline shift |
| 4 | PCNSL | 1 | 38 mm | Right third ventricle | Important | Homogeneous | Hypo | Hyper | Extension of Lesion to the Left |
| 5 | PCNSL | 1 | 22 mm | Left capsule- lenticular | Mild | Peripheral Heterogeneous | Hypo | Hyper | None |
| 6 | PCNSL | 1 | 44 mm | Left anterior paraventricular | Moderate | Homogeneous | Hypo | Hyper | None |
| 7 | PCNSL | 1 | 19 mm | Left parietal | Minimal | Homogeneous | Hypo | Hypo | none |
| 8 | PCNSL | 3 | 41 mm | Left frontal | Mild | Heterogeneous | Hypo | Hyper | None |
| 7 mm | Right parietal | Moderate | Heterogeneous | Hypo | Hyper | None | |||
| 9 | PCNSL | 1 | 19 mm | Left parietal | Minimal | Homogeneous | Hypo | Hyper | Calcification |
| 10 | PCNSL | 1 | 29 mm | Right thalamus | Mild | Absent | Hypo | Hyper | Hemorrhagic Component |

Patients were considered as having PCNSL when they had brain biopsy-proven CNSL and PET, or total body scan showing the absence of any abnormal growth elsewhere outside the CNS, as shown in Fig. (3) and Table 3. Out of all the patients diagnosed with lymphoma (N=10), only 1 patient (10%) was found to have peripheral lymphoma involving cervical, iliac, and diaphragmatic lymph nodes with secondary involvement of the CNS, and thus was diagnosed as having SCNSL. The remaining 9 patients (90%) were diagnosed as PCNSL. Out of all the 9 patients, 8 patients had the brain biopsy and PET scan (which did not show an abnormal uptake of radio-tracer in other parts of the body), and 1 patient had the brain biopsy and CT of the chest, abdomen and the pelvis with contrast (which did not show an abnormal lesions elsewhere outside the CNS).
In addition, results showed the classification of patients with PCNSL into either immuno-compromised or Immunocompetent (IC). All the 10 PCNSL patients were IC and this was confirmed by the absence of a history of post organ transplant immunosuppression, absence of congenital ID disorders, and a negative Human Immune-Deficiency Virus (HIV) test.
Finally, all CNSL were evaluated for different MRI and CT features/parameters, concerning the location, number of lesions, size, post-contrast enhancement, and presence of hemorrhage, necrosis, or calcifications.
Out of all the 9 IC-PCNSL patients, 8 patients had a supratentorial lesion (88.9%) and 1 had a solitary infratentorial lesion (11.1%), with homogeneous enhancement, where 7 of the supratentorial were solitary and only 1 was multi-focal with heterogeneous enhancement, as shown in Fig. (3) and Table 3. Out of all the supratentorial solitary lesions, 4 showed homogeneous enhancement, 2 showed heterogeneous enhancement, and 1 showed no enhancement. The results of MRI scans are summarized in Table 3.
4.5. Results of the Parameters of Immunocompetent (IC)-PCNSL Patients
Only IC patients with PCNSL were included in the study (N=9). These PCNSL patients’ results included the gender and the lesion location.
4.5.2. Lesion Location in IC-PCNSL Cases
The percentage of supratentorial lesions was found to be 90%. Using the Chi-Square test of independence, there was no statistically significant difference in what has been found in this study for location, compared to that described in literature review with a p-value of 0.527, i.e. p-value > 0.05.
Another significant finding was the presence of an isolated infratentorial cerebellar lesion in an immunocompetent patient with PCNSL.
5. DISCUSSION
This study involves the evaluation of the CT and MRI features of 10 histopathologically confirmed cases of CNSL in immunocompetent patients. In addition, the study compares the findings to those described in the literature for CNSL.
In principle, this study is in agreement with the previous results regarding the appearance of PCNSL and SCNSL on CT and MRI images. However, in the current study, there are two variables that were different to those described in the literature. The first one concerns the male-to-female ratio in IC-PCNSL patients, according to Bath et al., in all available studies, there was a slight male predilection with a ratio of 1.2 to 1 [3]. However, in this study, females were 5.6 times at a higher risk of developing PCNSL. The latter result was statistically significant with a p-value of 0.0162<0.05.
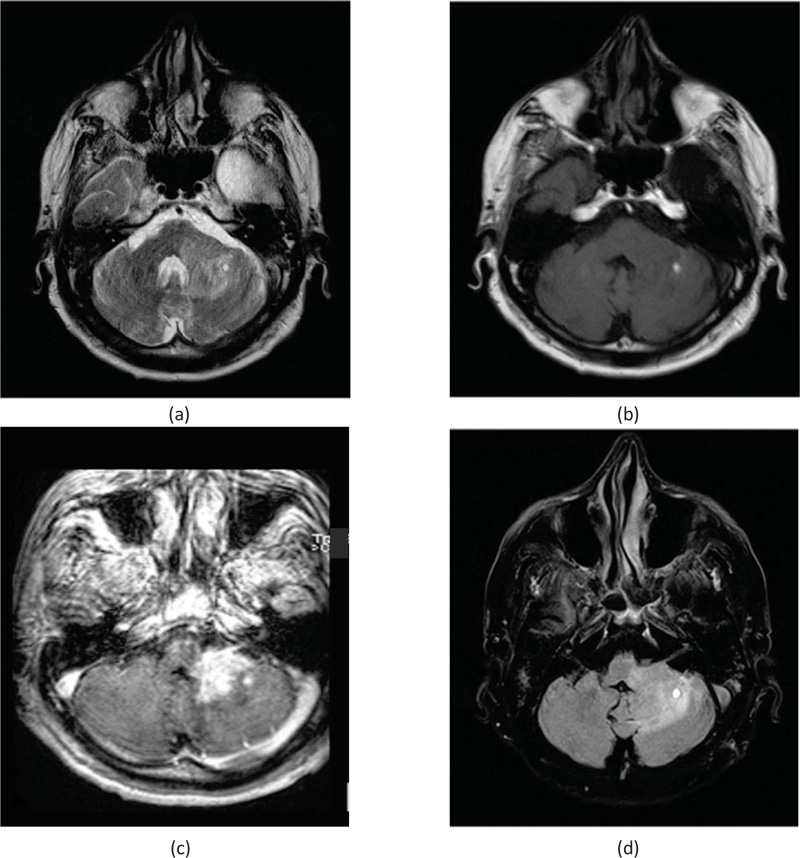
The second important and significant difference was the presence of an isolated Cerebellar lesion in IC-PCNSL patient. This finding was not described by any of the reports from Asian countries [10], as shown in Fig. (4).
Regarding the age explored in this study, the median age at diagnosis was 56 years with a range of 49 to 75 years, which was lower than that in previously reported data (median age: 65 years [11]).
Regarding the gender feature and according to Bath et al. and Brandao et al., the male predominance is generally recognized at a ratio of up to 1.2-1.7 [3, 12] in PCNSL, however, the data of this study showed female predominance with a ratio of 4 to 1, this finding was statistically significant with a p-value < 0.05.
Concerning the percentage of lymphoma among other brain neoplasms, of all patients administrated to ED, this study showed that lymphoma patients represented approximately 5%, which was in accordance with the data described in previously published reports [13].
Concerning the type of lymphoma, there is no statistically significant difference between what has been reported in this study and that described in the literature (Primary lymphomas represent 70-90% of all CNS lymphomas) concerning the distribution of patient according to type of CNS lymphoma (p-value = 0.263).
Concerning the gender of PCNSL of patients, there was female predominance and a nearly statistical significance with a p-value of 0.057 compared to the literature review. According to Tomita et al., the male to female ratio was 1.2/1 in IC-PCNSL patients [14], however, in this study the ratio was 3.5/1.
As for the location, in this study, the majority of lesions were supratentorial (90%), as shown in Fig. (5). This was in concordance to the location results described in the literature for CNS lymphoma. The majority of studies showed a supratentorial predominance of 70-90% [3, 6, 8, 15].
Pertaining to the number of lesions, the data showed concordance with that described for PCNSL, with 89% of lesions being solitary, as shown in Fig. (6). This percentage was slightly higher than that described in other studies 75% [16, 17], but the difference was not statistically significant with a p-value of 0.527, i.e. p-value > 0.05.
As for the enhancement pattern, the data and previous studies concur in that enhancement is most commonly homogeneous, as shown in Fig. (6). The percentage was 66.67%, i.e., lower than that reported in the existing studies 85-90% [18], however, this was not statistically significant [18].
Brain MRI showed left cerebellar lesion measuring 35 × 24 × 22 mm extends into the left middle cerebellar peduncle, the lesion appeared as a hyper signal on T2, as shown in Fig. (4a), and appeared as a hyposignal on T1, as shown in Fig. (4b). This mass enhanced in a nodular fashion after injection of a contrast agent on T1, as shown in Fig. (4c). Moreover, the lesion appeared as a hypersignal on FLAIR, as shown in Fig. (4d). It is note worthy that the presence of nodular intralesional image measuring 6 mm, showcased a hypersignal on T1 and T2, which was consistent with calcifications or minimal intralesional hemorrhage.
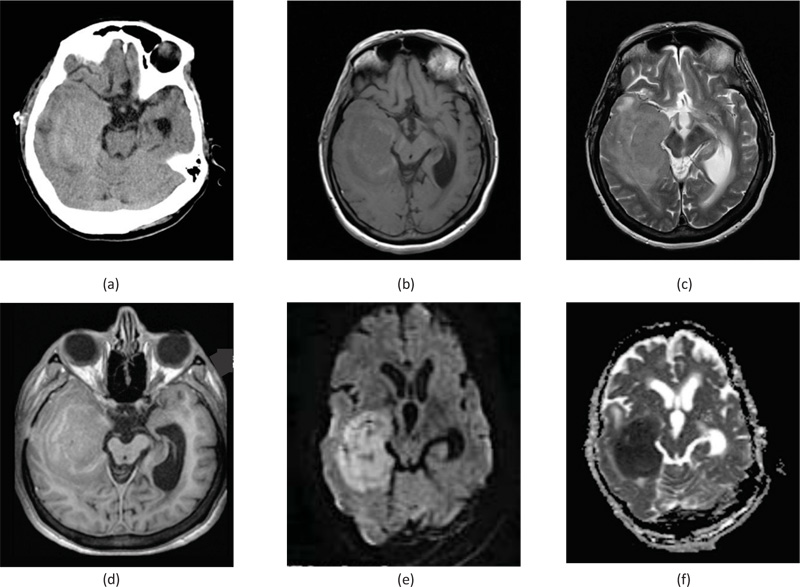
Brain CT in Fig. (5a) showed a right temporal lobe hyperdense lesion measuring 62× 34 mm, causing obliteration of the right temporal horn. MRI of the brain showed the presence of expansive mass at the level of the right temporal and parietal regions, showing a hyposignal with respect to grey matter on T1 in Fig. (5b) and on T2 in Fig. (5c). The lesion showed a restricted diffusion that appeared as a hyper signal on DWI, as shown in Fig. (5e), and hyposignal on the Apparent Diffusion Coefficient (ADC) Fig. (5f). The lesion shows heterogeneous ring enhancement after injection of contrast Fig. (5d). Absence of intralesional hemorrhage, calcifications, or necrosis. This lesion was proven to be PCNSL.
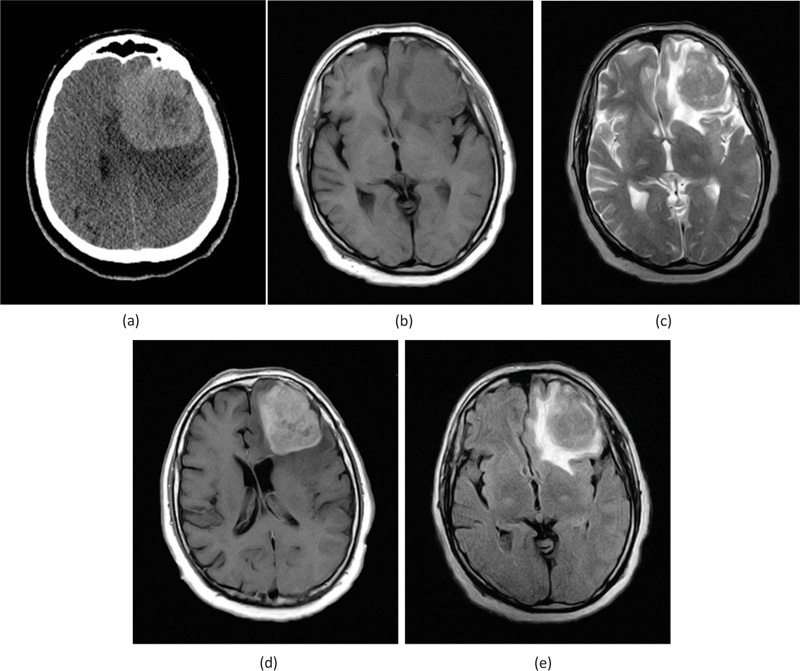
However, brain CT, in Fig. (6a), showed a large hyperdense frontal lesion on the left side, surrounded by moderate perilesional edema. Brain MRI showed the presence of anterior frontal lesion on the left side, measuring 65 mm with moderate perilesional edema, causing mass effect and displacing the mid-line structures towards the right. The lesion appeared as a hyposignal on T1, as shown in Fig. (6b), and appeared as a hypersignal on both T2 as shown in Fig. (6c) and FLAIR, as shown in Fig. (6e) with respect to the grey matter. The lesion showed nodular and homogeneous contrast enhancement on T1, as revealed in Fig. (6d), in the absence of hemorrhage, necrosis, or calcifications.
6. STUDY LIMITATIONS
Although this exploratory study has achieved its main aim, it witnessed several unavoidable limitations. The research focused on one region in Lebanon. Had the study covered a larger scale, its impact would be more recognizable. Moreover, a small PCNSL size of the sample has been reached due to the fact that CNSL is a very rare tumor.
CONCLUSION
The study of the CT and MR imaging data of 10 patients of CNSL in IC patients concurred with the previously described patient’s populations, except for gender. Tumors were mostly present in the 5th or 6th decade, and they can be solitary or multi-focal. Lesions are typically located in the supratentorial compartment. On brain CT, the lesions were hyperdense, and in pre-contrast MRI images, the lesions appeared hypointense on T1 and hyperintense on T2, but hypointense with respect to the grey matter. Furthermore, the lesions were surrounded by a mild to moderate edema, as compared to other intracranial neoplasms, such as glioblastomas. In post-contrast MRI imaging, the majority of lesions exhibited a homogeneous enhancement, heterogeneous ring-like enhancement was less common, and absence of enhancement was uncommon. Calcifications, hemorrhage, and necrosis were rare findings and were present in only one patient, when present, they were usually indicative of the presence of other diseases. Although CNSL has characteristic imaging findings on traditional imaging modalities like CT and the classical MRI, none of these provided unequivocally a differentiation between CNSL and other brain tumors. However, the new advanced MRI techniques have successfully identified the characteristic findings and parameters in CNSL that added a value in the differentiation of CNSL from other brain tumors.
FUTURE WORK
Researchers all over the world are currently intensively investigating the potential importance of advanced medical imaging, such as PET imaging and its tracers, as well as the new MRI contrast agents to reveal the important aspects of tumor biology. This research provided new insights that would substantially improve the preoperative diagnosis of CNSL. In the future, improved advanced imaging techniques may provide a non-invasive and accurate diagnosis, thus potentially changing the management algorithm from neurosurgical resection to surgical biopsy, before the initiation of chemotherapy and radiotherapy. Thereby, new non-surgical therapeutic measures could be considered. Newer imaging techniques could presumably play a pivotal role in the planning of new targeted therapies, prognosticators, and monitoring treatment response. Moreover, the exploration of improved imaging techniques may provide not only non-invasive and morphological characteristics but also non-invasive biological characteristics, and thus, accurate diagnosis could be considered. Also, the number of the lymphoma cases to be explored could be extended to include a wider database.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
Not applicable.
CONSENT FOR PUBLICATION
Informed consent was taken from all the patients when they were enrolled.
AVAILABILITY OF DATA AND MATERIALS
The data used to support the findings of this study are included in the article.
FUNDING
Declared none.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The authors would like to thank the unknown reviewers for their valuable feedback.


