All published articles of this journal are available on ScienceDirect.
Diffusion Tensor Imaging: A Promising New Technique for Accurate Identification of the Stria Medullaris and Habenula
Abstract
The Stria Medullaris (SM) is a white-matter tract that contains afferent fibres that connect the cognitive-emotional areas in the forebrain to the Habenula (Hb). The Hb plays an important role in behavioral responses to reward, stress, anxiety, pain, and sleep through its action on neuromodulator systems. The Fasciculus Retroflexus (FR) forms the primary output of the Hb to the midbrain. The SM, Hb, and FR are part of a special pathway between the forebrain and the midbrain known as the Dorsal Diencephalic Conduction system (DDC). Hb dysfunction is accompanied by different types of neuropsychiatric disorders, such as schizophrenia, depression, and Treatment-Resistant Depression (TRD). Due to difficulties in the imaging assessment of the SM and HB in vivo, they had not been a focus of clinical studies until the invention of Diffusion Tensor Imaging (DTI), which has revolutionized the imaging and investigation of the SM and Hb. DTI has facilitated the imaging of the SM and Hb and has provided insights into their properties through the investigation of their monoamine dysregulation. DTI is a well-established technique for mapping brain microstructure and white matter tracts; it provides indirect information about the microstructural architecture and integrity of white matter in vivo, based on water diffusion properties in the intra- and extracellular space, such as Axial Diffusivity (AD), Radial Diffusivity (RD), mean diffusivity, and Fractional Anisotropy (FA). Neurosurgeons have recognized the potential value of DTI in the direct anatomical targeting of the SM and Hb prior to Deep Brain Stimulation (DBS) surgery for the treatment of certain neuropsychiatric conditions, such as TRD. DTI is the only non-invasive method that offers the possibility of visualization in vivo of the white-matter tracts and nuclei in the human brain. This review study summarizes the use of DTI as a promising new imaging method for accurate identification of the SM and Hb, with special emphasis on direct anatomical targeting of the SM and Hb prior to DBS surgery.
1. INTRODUCTION
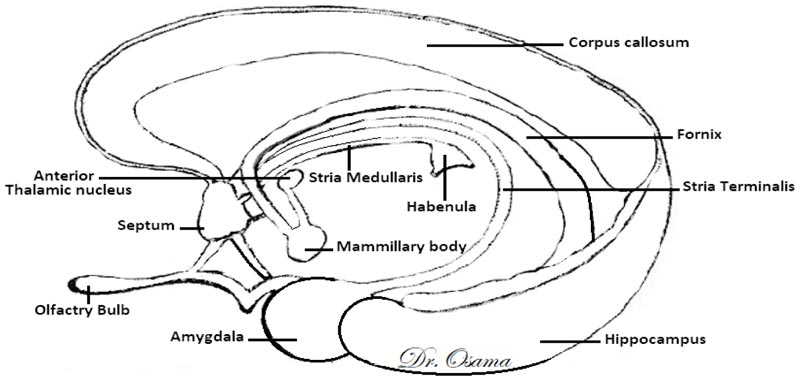
The Dorsal Diencephalic Conduction System (DDCS) is a highly conserved integrative and modulatory pathway that is present in all vertebrates. The DDCS is a pathway that transmits information from the cognitive-emotional forebrain to the regulatory midbrain areas. It is composed of three structures: the Stria Medullaris (SM), the Habenula (Hb), and the Fasciculus Retroflexus (FR) [1] (Fig. 1). In this conduction system, fibres from multiple frontolimbic areas form a single unidirectional tract known as the SM, which is a discrete epithalamic white-matter tract that emerges caudal to the anterior commissure, arches posteroinferiorly over the inter-thalamic adhesion, and terminates at both FR lateral and medial Hb nuclei [2, 3] (Fig. 2).
In mammals, the habenular complex comprises two separate nuclei on each side: a medial nucleus (MHb) and a lateral nucleus (LHb) [4]. The Hb, in turn, projects down through the fasciculus retroflexus and influences midbrain monoamines of the raphe nuclei and ventral tegmental area [5]. The habenular nuclei receive input chiefly through the SM [6]. The Hb outputs either directly or indirectly to midbrain’s monoaminergic nuclei and affects the release of serotonin [7], norepinephrine [8], and dopamine [9].
Activation of Hb reduces monoaminergic output [10], and the Hb has been implicated in aversive decision making [11, 12], pain [13], reward pathways [14], and mood [15, 16]. Hb changes have also been found in disorders such as depression [17, 18] and chronic pain [19]. The flow of information from the limbic frontal regions to the Hb through the SM, as well as the relationship between neurotransmitter release and habenular activity, has heightened interest in the study of the role of the SM and FR in neuropsychiatric disorders such as schizophrenia, depression, bipolar disorder, addiction, and chronic pain.
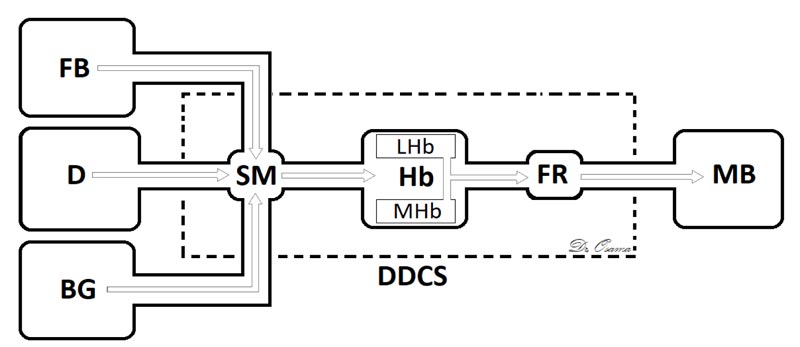
Hb receives, through the SM, significant input from forebrain regions, including many limbic and dopamine-rich structures [20] and, through FR, it interconnects with the ventral tegmental area, the interpeduncular nucleus, with dopamine-containing neurons in substantia nigra, and is a major input to the raphe nuclei [21]. The habenular nucleus is unusual in that it shows a substantial inhibition of glucose metabolism and blood flow during the administration of dopamine agonists [22] or rewarding brain stimulation [23]. It apparently mediates part of the negative feedback of dopamine agonists onto dopamine cells, for lesions of the Hb or SM markedly attenuate methamphetamine-induced inhibition of substantia nigra cells [24]. The finding that continuous amphetamine and cocaine have markedly dissimilar effects in caudate but very similar neurotoxic effects in lateral habenula and fasciculus retroflexus suggest that alterations in these pathways may at least partially underlie the very parallel effects which develop during amphetamine or cocaine binges in humans, i.e., progressive paranoia followed by a rebound depression [25].
Midbrain neurons are an essential component of the circuitry underlying motivation and reinforcement. They are activated by rewards or reward-predicting cues and inhibited by reward omission. LHb forms reciprocal connections with midbrain dopamine neurons, shows the opposite response being activated by reward omission or aversive stimuli and inhibited by reward-predicting cues. It has been hypothesized that habenular input to midbrain dopamine neurons is conveyed via a feedforward inhibitory pathway involving the GABAergic mesopontine rostromedial tegmental area [26]. Brown et al. showed that exposing rats to low intensity footshock (four, 0.5 mA shocks over 20 min) induces cFos expression in the rostromedial tegmental area and that this effect is prevented by lesions of FR. It is particularly interesting that the selective degeneration seen with nicotine administration occurred in the same nuclear complex and tract that degenerates with chronic dopaminergic stimulant administration, although the dopaminergics affect the lateral habenula and mantle of the FR [25].
Due to a lack of advanced imaging techniques for accurate assessment of SM in vivo, no study has been done to investigate its role in these diseases. However, the advent of DTI and fibre tractography has revolutionised the field of non-invasive imaging of white matter connectivity of the brain and improved the understanding of many neuropathologic and psychiatric disorders. DTI has mostly been used to evaluate microstructural changes in the brain by measuring the motility of water molecules in tissue [27]. Its imaging capabilities are based on the ability to determine the orientation and diffusion characteristics of white matter [28].
Recent advances in DTI tractography have allowed for the identification of white-matter tracts with ever-greater precision [29, 30]. This technique has proved its importance in the presurgical mapping of white-matter tracts before intracranial surgical procedures. It has been particularly useful for identifying large white-matter tracts such as the superior longitudinal fasciculus, inferior longitudinal fasciculus, and corpus callosum [31]. It provides indirect information about the microstructural WM architecture and its integrity in vivo, based on water diffusion properties in the intra- and extracellular space [32].
The SM is a good prospective candidate for tractography because it does not deviate or branch from its origin (behind the anterior commissure) to its termination (the habenula). However, it is challenging to render it accurately with DTI tractography because it is short, thin, and highly curved. Furthermore, it is imbedded deep within brain tissue, so it is not easy to isolate from the surrounding white matter structures. The SM and its termination, the Hb, are inconsistently seen in standard T1/T2 MRI as a result of being “missed” between slices [33, 34].
The application of DTI tractography for accurate imaging of the SM could lead to a greater understanding of psychiatric neuropathology, addiction, and pain disorders through the analysis of its diffusion metrics. The SM is a target for Deep Brain Stimulation (DBS) in the treatment of severe depression because of its pivotal role in integrating diverse basal forebrain limbic regions into a single tract and relaying directly with the Hb [35, 36]. DTI analyses of the afferent and efferent pathways of Hb, including SM, can be used as an imaging marker for diagnosis of various psychiatric disorders. DBS is a minimally invasive and reversible method that is widely used to treat various neurological and psychiatric disorders by stimulating structures that are located deep in the brain [37].
To date, DBS of the Hb has been described in only two patients, but the results have been promising [38]. Although direct targeting of the Hb is feasible, its size and location mean that meticulous planning is required to both effectively target the structure and avoid side effects [39]. The aim of this review study is to summarize the use of DTI as a reliable method for direct anatomical targeting of the SM and the Hb prior to DBS surgery. Special emphasis is placed on the technical details of preoperative probabilistic DTI done prior to DBS surgery due to its importance and effectiveness in direct anatomical targeting of the SM and Hb.
1.1. MRI Acquisition
Kochanski et al. [34] conducted a study in Chicago, Illinois, to determine whether DTI could be used to define the SM. Pre-operative DTI was performed on five patients at one to two weeks prior to a scheduled DBS implantation procedure using a 3T MRI scanner. Their pre-operative DTI protocol included a localizer, thin-slice coronal and axial T2-weighted turbo spin-echo sequences, a 3D gradient echo sequence for Susceptibility-Weighted Imaging (SWI), and a post-contrast 3D T1-weighted sequence for neuro-navigation using a Magnetization Prepared Rapid Gradient Echo (MPRAGE) sequence. MPRAGE images were used as a reference for the correction of motion and distortion artefacts for diffusion images.
A diffusion-weighted sequence was acquired before the administration of the contrast agent, and a parallel imaging acceleration factor of 3.0 was used to mitigate susceptibility distortions. Furthermore, 65 contiguous axial slices with a thickness of 2 mm were used to provide whole brain coverage. The Hb receives its main input from different brain regions through the SM, so it was hypothesized to be an opportune seed region for the application of probabilistic tractography. On three consecutive 1.2-mm slices of axial T1-weighted MPRAGE images, the Hb was identified by its morphological features, in which it appears as a triangular ridge extending into the third ventricle on the medial surface of the thalamus [33], [39, 40]. This visibility facilitated manual drawing of a two-dimensional seed region within the central axial slice of the Hb on high-resolution MPRAGE images.
A single operator drew seed regions for all subjects (10 total seed regions) in the “FLSView” module of FSL [41]. A spacing of one voxel was maintained between tissue and CSF from the perceived interface. Colorized probabilistic tractograms were overlaid on the original MPRAGE images. A second study was also done to determine whether the Mass Intermedia (MI) serves as a conduit for the crossing of limbic fibres such as the SM [42]. Probabilistic DTI was applied to 10 patients with MI seen in MRI. The SM was reliably seen in all 10 subjects with evidence that the SM fibres crossed to the ipsilateral hemisphere.
Another study was done by Roddy et al. [5] at Trinity College Dublin, Ireland. They proposed a method of deterministic Diffusion-Weighted Imaging (DWI) tractography based on Constrained Spherical Deconvolution (CSD) for reconstructing the SM in vivo. They also studied the effects of gender and age on tract diffusion metrics. DWI was applied to 50 healthy participants, and the reproducibility of the method was evaluated through inter-rater comparisons. Their pre-processing steps included (a) the exporting and standardization of diffusion output files, (b) correction of signal drift, (c) correction of Gibbs ringing artefacts, (d) manual glyph inspection to check the orientation and directionality, (e) rigid body motion correction to correct head motion artefacts and affine registration to correct eddy current artefacts, and (f) affine registration to correct EPI deformation.
In Explore DTI, CSD was applied to generate whole brain tractography. The voxel size for whole-brain seeding was 2 mm3, and the threshold of fibre orientation distribution was 0.1. Multiple random seed placements were applied for the achievement of whole brain tractography with one seed per voxel. For each subject, two separate files of whole brain tractography were generated. The first one was only used for placement of the initial gate and to exclude large structures such as the fornix. It had few streamlines and was applied for simply scouting out the SM region. The second one was created for the reconstruction of complex tracts with streamlines of high density.
The step size for low-fidelity tracts of the whole brain was set at 1 mm, and the maximum angle threshold was set at 45°, while the step size for high-fidelity tracts of the whole brain was set at 0.5 mm, and the maximum angle threshold was set at 89°. The lower threshold was selected because the SM was expected to be larger than 10 mm, and the higher threshold was selected for the reproduction and removal of larger tracts around the SM, such as the stria terminalis [43], fornix [44], superior thalamic peduncle [45], and thalamocortical radiations [46]. The SM was isolated using a Boolean logic protocol with the application of OR and AND gates. Neurons of the SM blend into a solitary tract posterior to the anterior commissure, so the upper part of the commissure was used as a landmark for anterior axial OR gate placement.
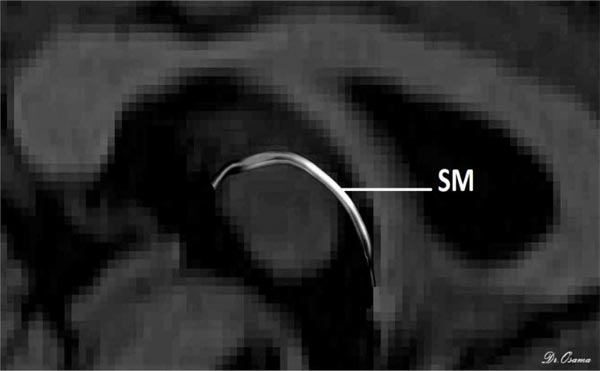
The posterior end of the SM lies bilaterally in both habenulae within the stalk of the pineal gland, which is clearly seen in T1 imaging, so the uppermost point of the pineal gland was established as the primary landmark for the placement of the posterior OR gate. Finally, as the SM arches above the inter-thalamic adhesion between both thalami, the inner halves of both thalami were used as the primary landmark for AND gate placement. The SM was perfectly generated for all 50 participants and reconstructed with excellent inter-rater reliability for dimensions and diffusion metrics in every participant. There was a positive correlation of the Radial Diffusivity (RD) with age, but there was no effect of gender on the diffusion or dimension metrics (Fig. 3).
Strotmann et al. [33] investigated the feasibility of discriminating the habenula in human brain using high-resolution structural MRI and diffusion-weighted imaging at 7 Tesla (T). To allow precise localization of the region of interest, they first acquired a 3D T1-weighted data set using the MP2RAGE sequence (TR = 6000 ms, TI1 = 900 ms, TI2 = 2750 ms, TE = 3.11 ms, 0.7 mm isotropic resolution, flip angle 1 and 2 = 4° and 3°). DW images were acquired with optimized Stejskal-Tanner diffusion weighting scheme [29] and a novel technique, a combined approach of reduced FOV acquisition (zoomed imaging) and parallel imaging (GRAPPA), given the name ZOOPPA (Zoomed Partially Parallel Acquisitions), was applied [19]. To solve the problems of susceptibility artifacts and image blurring in high-resolution diffusion MRI at ultra-high field strength, the acquisition was accelerated using parallel imaging [30]. However, for this high resolution and field strength, it is necessary to achieve high acceleration factors with a minimum amount of additional noise, arising from the parallel image reconstruction. A combination of zoomed imaging [31, 32] and parallel imaging as described by Heidemann et al. [33] can be used to significantly improve the image quality of single-shot EPI acquisitions. This method is beneficial for high resolution DWI at ultra-high field strength [19]. They used a protocol with 1 mm isotropic resolution with the following parameters: TR = 10400 ms, TE = 82 ms, FOV = 144 × 150 mm2, image matrix 144 × 150, 75% partial Fourier, total acceleration factor 4.2. Fat suppression was performed with a combination of the gradient reversal method [34] and a low SAR approach [35] as described by Eichner et al. [36]. Diffusion weighting was performed along 60 diffusion-encoding gradient directions with a b-value of 1000 s/mm2. Seven images with no diffusion weighting were placed at the beginning of the sequence and after each block of 10 diffusion-weighted images, to provide an anatomical reference for offline motion correction. The interleaved measurement of 71 slices with 10% overlap covered most of the brain in the anterior–posterior direction. Four averages were acquired, resulting in a total acquisition time of 48 min for 71 slices. Multiple orientations of crossing fibers were modeled using constrained harmonized spherical deconvolution (SCD), and probabilistic streamline tractography methods were applied [47]. Probabilistic tractography takes into account intra-voxel crossing fibers for estimating the pathways that originate at any given seed voxel and provides quantitative information about the probability of structural connectivity that a white matter tract will pass through any other voxel in the brain [48]. Probabilistic tractography showed the two major fiber bundles that connect habenular nuclei with limbic forebrain structures and brainstem. Streamline tractography relies on a rank-2 tensor (3×3 matrix) model of a voxel’s diffusion field, where the diffusion field is defined as displacements of protons along the directions in 3D space averaged over a given time [49, 50]. With ultra-high-resolution data, more fiber orientation distribution parameters have to be estimated than actually measured. For this reason, spherical harmonized deconvolution can resolve orientations with smaller angles, and provide a robust estimation of the distribution of fiber orientations in each voxel while preserving angular resolution [33].
2. DISCUSSION
Despite the importance of the SM and Hb in neuropsychiatric pathologies, there is a paucity of studies discussing the application of DTI in SM and Hb imaging. Kochanski et al. conducted two related studies, and one was done to determine whether DTI could be used to define the SM [34]. They applied presurgical probabilistic DTI to define the estimated location of the Hb as a seed point. However, the application of a single probabilistic seed point with a local approach in this study may have suffered from limitations in capturing the SM in its known gross anatomical location, and an approach based on a local seed is vulnerable to errors with the propagation of the tract. The study found that probabilistic tractography reliably visualized the SM bilaterally in all five patients. Their second study was done in 2018 to determine whether the Mass Intermedia (MI) serves as a conduit for the crossing of limbic fibres such as the SM [42]. Probabilistic DTI was applied to 10 patients with MI seen in MRI. The SM was reliably seen in all 10 subjects with evidence that the SM fibres crossed to the ipsilateral hemisphere.
Another study was done by Roddy et al. [5], who applied an anatomically guided three-gated approach to whole-brain tractography to predefine the start and end points of the SM to facilitate its comparison in future studies. The advantage of this approach is that it describes the trajectory of the SM more accurately, and it could be important in perioperative localization of the SM for DBS. It may also assist in the 3D stereotactic localization of the SM prior to electrode placement in the future, as well as provide another layer of anatomical certainty in association with standard imaging during electrode insertion. The SM was perfectly generated in all 50 participants and was reconstructed with excellent inter-rater reliability for the dimensions and diffusion metrics in every participant. There was also a positive correlation of Radial Diffusivity (RD) with age, but there was no effect of gender on the diffusion or dimension metrics.
It has been found that DTI of the SM and Hb is subject to some technical challenges, such as the high curvature of the SM in a relatively narrow space that is deep within the brain tissue. This challenge was solved by using a high angle and small step size in the SM reconstruction. Another challenge is that the SM lies in close proximity to other white matter bundles, such as the fornix and stria terminalis. It has been found that CSD could solve this challenge, and it was chosen as the most appropriate tractography technique due to its superior ability to detect complex crossing and kissing fibres in a recent analysis of fibre estimation approaches [50]. Furthermore, the generated SM in DTI reconstruction only describes the diffusivity in the region of interest, and it is assumed that it corresponds to an actual tract. To consistently encompass as much of the SM as possible, the initial gates should be broad, resulting in plenty of time for clean-up.
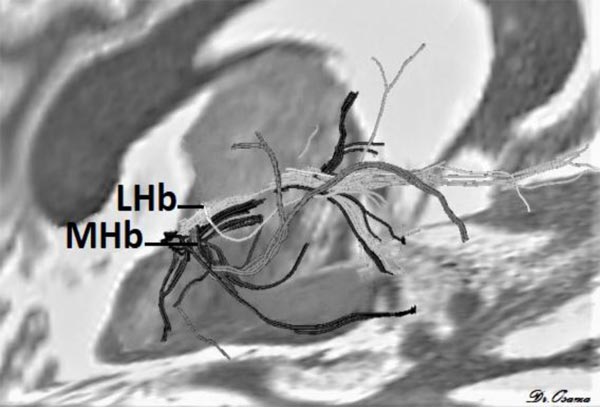
Strotmann et al. [33] concluded that high-resolution DWI at 7T MR can provide sufficient signal-to-noise and contrast to enable identification of the LHb and MHb. Streamline tractography applied at 7T MR showed that MHb tracts pass to the brainstem, while LHb tracts go to the forebrain (Fig. 4) it can also detect major fiber tracts that connect Hb with other brain areas.
CONCLUSION
This study has described DTI as a promising new imaging method for accurate localization of the SM and Hb. DTI facilitates imaging of the SM and Hb and provides insights into their properties through the investigation of their monoamine dysregulation. DTI has received heightened interest for the stimulation of the limbic system’s white-matter tracts, and a single probabilistic seed point with a local approach is a reliable probabilistic tractography technique for defining the SM and Hb prior to DBS. Due to the promising results of the application of DTI in direct anatomical targeting, broader targeting of the lateral Hb via the SM in the future could provide a more anatomically favourable target for DBS. DTI represents a new light in the dawn of neuropsychiatric management and research. Enhancement of DTI acquisition techniques by decreasing scanning time, increasing signal-to-noise ratio, and the implementation of high spatial resolution will assure the utilization of DTI as an accurate diagnostic tool, and it could even be applied in clinical research.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


