All published articles of this journal are available on ScienceDirect.
Unusual Intracranial Manifestation of Infective Endocarditis
Abstract
Objective:
The mycotic aneurysm is a rare intracranial pathology seen with pre-existing infective endocarditis. It has a high mortality rate due to its risk of rupture and needs early diagnosis and treatment.
Methods:
A 23-year male patient who presented with infective endocarditis subsequently developed a left parietal-temporal intracranial haemorrhage with suspicion of aneurysm after the course of antibiotic treatment as seen on Computed Tomography (CT) scan. Digital Subtraction Angiography (DSA) revealed a ruptured fusosaccular aneurysm in the distal parietal branches of the left Middle Cerebral Artery (MCA), for which glue embolization of the distal parent artery and aneurysm was done.
Result:
The interventional endovascular procedure was done with complete obliteration of the distal parent artery, mycotic aneurysm, and normal filling of the left internal cerebral artery (ICA) branches.
Conclusion:
Mycotic intracranial aneurysms (MIA) are a rare form of cerebrovascular pathology which needs early diagnosis with endovascular intervention when rupture occurs.
1. INTRODUCTION
Infectious intracranial mycotic aneurysms due to systemic infection or immunocompromised state cause direct bacterial invasion of the vessel wall. Ruptured aneurysms frequently present with intracerebral (intraparenchymal) hemorrhage (ICH) in contrast to pure subarachnoid hemorrhage (SAH), which is a characteristic of non-infectious, saccular aneurysms [1]. This makes the diagnosis of atypical-appearing aneurysmal pathology with rupture difficult. It can be mistaken for intraparenchymal hemorrhage with resulting delay in therapy. An intracranial mycotic aneurysm is an unusual cause of hemorrhage and a known complication of infective endocarditis. Early diagnosis and treatment with endovascular intervention is the best option to prevent further morbidity and mortality. We present a patient who was diagnosed using CT imaging and treated with DSA guided endovascular intervention.
1.1. Narrative
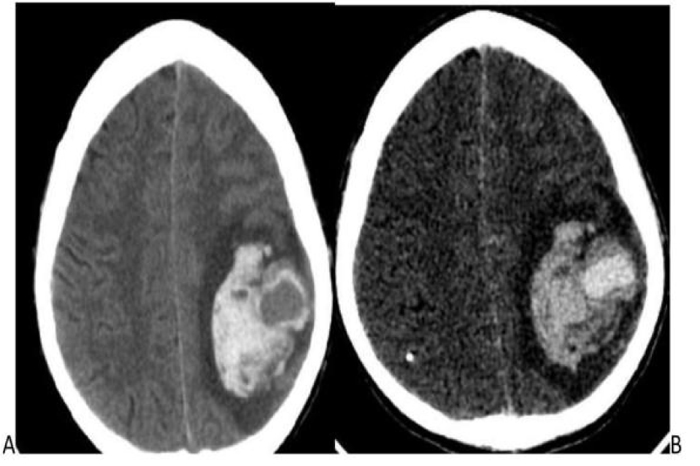
A 23 years male patient, diagnosed with infective endocarditis on antibiotics prior to admission, complained of fever, seizures, altered sensorium, and vomiting for 3 days. General physical examination was normal. The blood count was within normal limits, with Hb:8.9 g/dl, WBC: 8200 cells per Microliter, ESR: 140 mm/hr, and platelet count: 1,17,000 per microliter. There were no stigmata of an immune-compromised state. Ultrasound Echocardiography revealed mitral valve prolapse with mitral regurgitation and vegetations over the mitral area > 1cm. The patient was on a course of antibiotics for 3 weeks. Pre-discharge echocardiography showed a reduction in the size of the vegetation. One week later, the patient developed seizures and focal neurological deficits, for which the patient was admitted again. Clinical diagnosis of embolic infarct was suspected. The patient underwent CECT, which showed an intensely enhancing central area with surrounding intra-parenchymal hemorrhage in the left parietal-temporal lobe, most likely due to a ruptured mycotic aneurysm on correlating imaging and clinical findings (Fig. 1A and B). CT angiography (CTA) was withheld due to financial constraints. Informed consent was obtained for angiography and intervention.
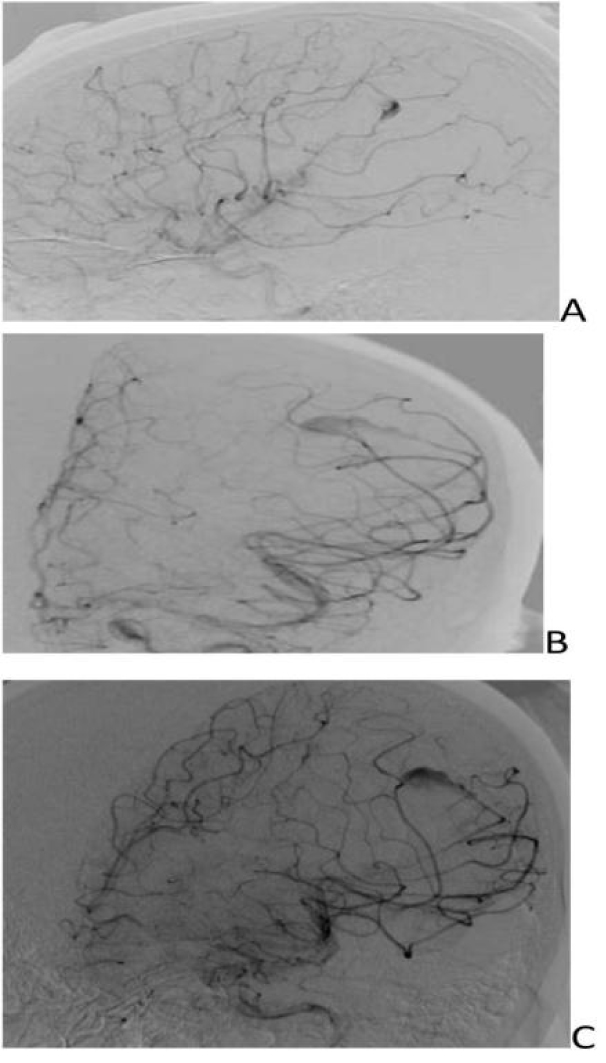
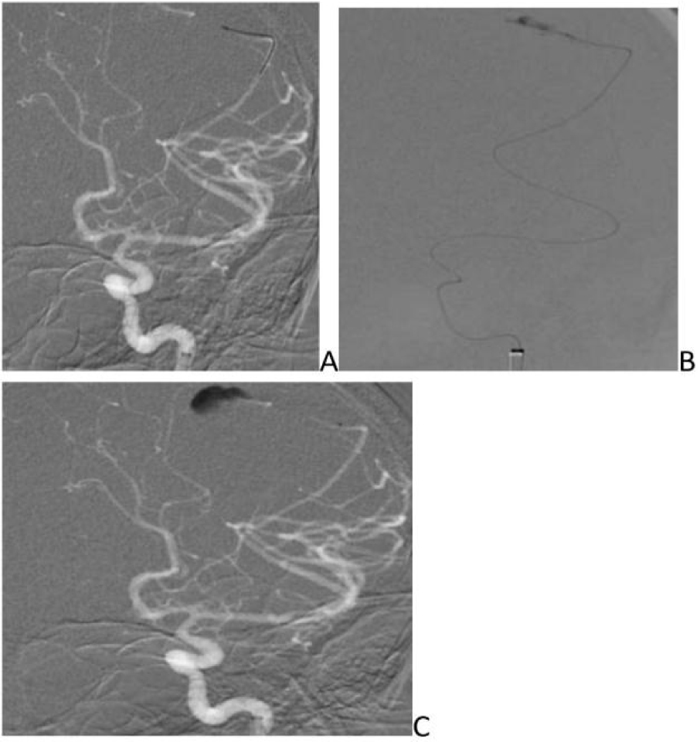
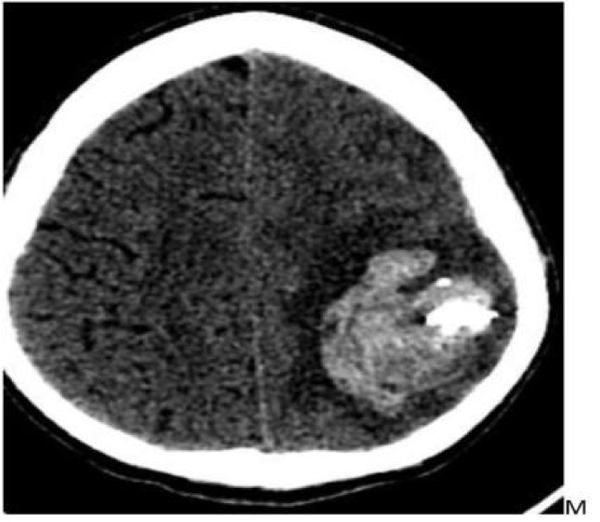
- DSA revealed fusosaccular aneurysm (11 x22mm) arising from the left MCA distal parietal branches projecting posterolaterally (Figs. 2A-C). Under general anesthesia (GA), 6F Neuron as guiding catheter was placed in the left MCA. Headway Duo microcatheter (167cm) (internal diameter at tip 0.013 inches, proximal 0.0165 inches with outer diameter distal 1.3F and proximal 2.1F with a length of 167cms) and Traxcess 0.014 micro guidewire (200cms length 40cm hydrophilic coated, 3cms radiopaque tip, distal outer diameter 0.012 inches or 0.30mm and proximal 0.014 inches or 0.36mm) were placed through guiding catheter up to the neck of the aneurysm, and distal parent artery and aneurysm were embolised using 62% histoacryl glue (Figs. 3A-C). Endovascular intervention with complete obliteration of the distal parent artery, mycotic aneurysm, and normal filling of the rest of the left ICA and MCA branches was accomplished (Figs. 4A-C). The post glue embolization check angiogram showed no filling of the aneurysmal segment or any other abnormal vascular changes. Follow-up CT showed a reduction in the size of the haemorrhage (Fig. 5).
The patient had uneventful intra- and post-procedural outcomes with normal neurological evaluation at discharge from the hospital.
2. PERSPECTIVE
Choice of endovascular treatment versus open surgery depends on the magnitude of factors described above with consideration to the morphology, location, with or without the sacrifice of the parent artery, whether the patient requires valve replacement surgery, and overall health status.

3. DISCUSSION
Mycotic intracranial aneurysms are a rare form of cerebrovascular pathology for which obliteration must be undertaken when they rupture or fail to respond to antibiotic therapy [2].
Invasion and inflammatory destruction of vessel walls secondary to septic emboli result in vascular dilatation and aneurysmal formation. These lesions are extremely friable due to their incomplete vascular wall and behave as pseudoaneurysms. The cerebral mycotic aneurysm has a mortality rate of up to 13%, and this increases to 80% in the case of rupture [3]. Their critical location, friability, and propensity to occur bilaterally result in an unpredictable risk of rapid neurological decline and death, making the timing and specific nature of treatment a unique dilemma faced by the treating physician [4].
The diagnosis of infectious intracranial aneurysms can be challenging as patients can be asymptomatic or present with subacute, nonspecific symptoms such as fever, headache, nausea, vomiting, chills, malaise, and minor focal deficits from intermittent shedding of septic emboli. In Lobar intraparenchymal hemorrhage, the review of source and Maximum Intensity Projection (MIP) images, including the delayed phase, should be performed to exclude the presence of connecting vessel, which favors the diagnosis of a ruptured mycotic aneurysm in clinically ambiguous presentations [5].
Since William Osler mentioned “mycotic aneurysms” in 1885, intracranial mycotic aneurysms (IMAs) have been widely reported. Historically, the management of IMAs has been based on limited data from literature reviews and case reports.
Several reviews on the management of IMAs have been sporadically reported [6]. Mycotic aneurysms have been reported < 10% of all intracranial aneurysms. This is mainly due to the fragile cardiac vegetation lodging in the intracranial vessels at the distal branches, followed by inflammation and necrosis of the vessel wall leading to the formation of pseudoaneurysm [7]. These aneurysms are thin-walled with wide/absent neck predisposing to rupture and consequent bleeding. The mortality rate is up to 80% if left untreated. Bacteria, especially streptococcus and staphylococcus organisms, are most commonly involved, followed by viral (HIV 1, varicella-zoster) and fungal aetiology (aspergillosis, candida). These infectious agents spread via the haematogenous route in the case of endocarditis or extravascular invasion of the arterial wall in an immune-compromised patient. The most common site for an infectious intracranial aneurysm is distal branches of anterior circulation (especially MCA) [7].
The most common clinical presentations are headache and fever, and the less common symptoms are seizures, ocular palsies, and focal neurological deficits. In the case of an extracranial aneurysm, a patient usually presents with painful pulsatile lateral cervical lesion and dysphagia /dysphonia; if left untreated, it leads to haemorrhagic shock [8]. The most common presentation is stroke (20-40%), followed by intracerebral haemorrhage (4- 27%) and the rare presentations are infectious intracranial aneurysm (2-4%), cerebral abscess (1-7%), and meningitis (1-20%) [9, 10]. Diagnosis of intracranial aneurysm is made by CTA, Magnetic Resonance Angiography (MRA), and DSA. The gold standard modality is DSA, but Multidetector CT (MDCT) CTA can be used in complete visualization of the vascular anatomy. In aneurysms <3mm, distal to the location with circumferential involvement of the vessels, CTA is less sensitive. Clinical symptoms and echo showing valve vegetations or occurrence of new pansystolic murmur and other general symptoms are to be considered.
The following criteria are used for making the decision regarding the interventional treatment: the known case of endocarditis; distal in location, involving segments M2, M3, or M4 of the middle cerebral artery or posterior cerebral artery (PCA); or proximal segments, involving M1 and PCA P1 in addition to at least two of the following criteria: (a) change in size or morphology of aneurysm on consecutive angiograms, (b) presence of any another intra-or extra-cranial aneurysm, (c) rupture of an aneurysm, (d) arterial occlusion or stenosis adjacent to aneurysm, (e) cerebral infarction due to occlusion of the arteries at the level of an aneurysm, and (f) recent neurological focal deficit associated with detection of new aneurysm detection. Conservative treatment is controversial, with outcomes being variable, in the following cases: a) In unruptured cases, b) High-risk patient especially when the aneurysm is in a proximal location where parent artery occlusion cannot be done with circumferential vessel involvement [11]. Surgical treatment is carried out in ruptured cases, especially in young patients with the risk of limited morbidity along with the presence of hematoma and mass effect [12]. Endovascular treatment is required in ruptured cases involving old patients with multiple comorbidities, where the aneurysm is dysplastic/absent aneurysmal neck, being candidates for cardiac surgery due to distal location of the aneurysm and multiple aneurysms. For mycotic aneurysm, endovascular treatments are considered appropriate due to advantages, including the reduced requirement for anesthesia, especially for patients with impaired valve function, rapid instillation of anticoagulant, and the reduced time period between cardiac surgery and aneurysm treatment. Endovascular treatments can be carried out via a direct approach and an indirect approach. The indirect approach involves occlusion of the parent artery using coils/liquid embolic agents, while the direct approach involves the use of coils, stent-assisted coiling, flow diverter, and liquid embolic agent [12].
CONCLUSION
Choice of endovascular treatment versus open surgery depends on the magnitude of factors described above with consideration to the morphology, location, with or without the sacrifice of the parent artery, whether the patient requires valve replacement surgery, and overall health status. Prognosis relies on prompt recognition and aggressive treatment. Both endovascular treatment and open surgery have been shown to increase the overall survival of the patient as compared to conservative management.
The patient's consent was taken before imaging and intervention procedures, and it was stated that scientific publication could be possible as per the outcome of imaging findings and intervention.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
No animals/humans were used for studies that are the basis of this research.
CONSENT FOR PUBLICATION
Not applicable.
STANDARDS OF REPORTING
CARE guidelines were followed for this study.
AVAILABILITY OF DATA AND MATERIALS
The data sets used during the current study can be provided from the corresponding author [A.M], upon reasonable request.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared None.


