All published articles of this journal are available on ScienceDirect.
Angiographic, CT, and MRI Findings in Moyamoya Disease: A Case Report with an Updated Review of the Literature
Abstract
Background
Moyamoya disease (MMD) is an occlusive cerebrovascular condition characterized by progressive stenosis of the terminal portion of the internal carotid artery (ICA) and the development of an abnormal vascular network at the base of the brain. This disease predominantly affects individuals in East Asian countries, with an incidence rate ranging from 6.03 to 9.1 per 100,000 people.
Case Presentation
We report the case of a 41-year-old Hispanic woman who presented severe headaches, nausea, vomiting, and intermittent loss of alertness over a 15-day period. Upon admission, her vital signs were normal, and no focal neurological deficits were observed. Initial plain CT imaging revealed an interhemispheric subarachnoid hemorrhage with intraventricular involvement in the occipital recess and right atrium. Subsequent angiographic CT with 3D reconstructions exhibited the classic 'puff of smoke' appearance indicative of Moyamoya disease. Perfusion-weighted imaging (PWI) demonstrated normal relative cerebral blood flow, blood volume, and mean transit time in both hemispheres. Based on these imaging findings, the patient was diagnosed with MMD. She underwent an indirect revascularization procedure known as encephaloduroarteriosynangiosis, which involved suturing branches of the superficial temporal artery to the dura.
Discussion
This case report underscores an atypical presentation of MMD in a Hispanic patient diagnosed by a combination of digital subtraction angiography (DSA), 3D CT angiography, and brain perfusion MRI. The findings highlight the importance of recognizing and diagnosing this rare condition in populations outside of East Asia. Furthermore, this report includes a review of the updated literature on MMD, providing valuable information on its diagnosis and management.
Conclusion
The clinical presentation and imaging findings, in this case, underscore the need for advanced diagnostic techniques, such as perfusion-weighted imaging (PWI) and quantitative color-coded parametric DSA (QDSA), to improve diagnostic precision and treatment planning. The successful application of indirect revascularization through encephaloduroarteriosynangiosis demonstrates the efficacy of surgical interventions in the treatment of MMD. Addressing ethnic disparities in MMD is crucial to improving early diagnosis and patient outcomes. Future research should focus on refining treatment algorithms, investigating nonsurgical interventions, and examining cognitive and psychological outcomes to further improve patient care.
1. INTRODUCTION
Moyamoya disease (MMD) is a chronic cerebro- vascular disorder characterized by progressive steno- occlusive changes in the terminal portion of the internal carotid artery (ICA) and the development of an abnormal vascular network at the base of the brain [1-4]. The term “moyamoya,” which means “puff of smoke” in Japanese, describes the appearance of this abnormal vascular network on angiographic imaging [1-3, 5, 6]. MMD is more prevalent in East Asian countries, such as Japan and Korea, compared to Western countries. In Japan, the incidence ranges from 0.94 to 1.13 per 100,000 inhabitants, with a prevalence of 5.22 to 10.5 per 100,000 people [7].
MMD is a significant cause of non-atherosclerotic stroke and transient ischemic attack (TIA) in regions with high prevalence, such as Japan and South Korea. The disease typically affects two main age groups: 10-20 years and 35-50 years, with a female-to-male ratio of approximately 2:1 [2, 3]. As the disease progresses, it can cause ischemic or hemorrhagic events due to blocked blood flow, resulting in high rates of disability and mortality [2, 3, 5, 6, 8]. The exact cause of MMD remains unknown [4, 7].
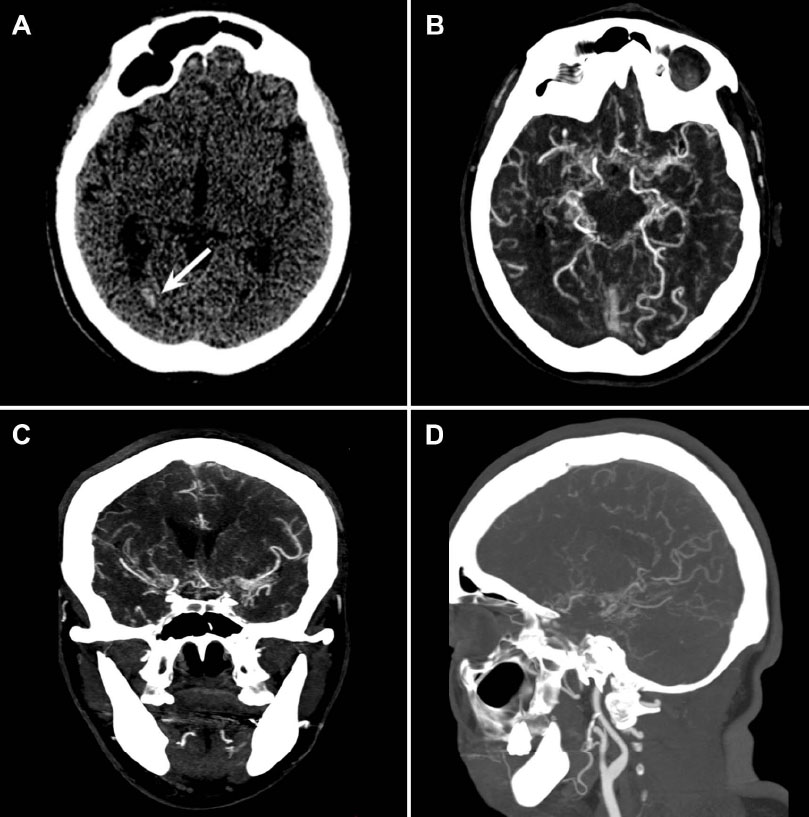
(A) Plain axial head CT showing interhemispheric subarachnoid hemorrhage with intraventricular extension into the occipital recess and right atrium. (B-D) Multiplanar reconstruction angiotomography. (B) Axial plane showing multiple collateral arteries throughout the cisterns, with numerous perforating branches corresponding to the lenticulostriate arteries. (C) Coronal plane illustrating the extensive collateral network. (D) Sagittal plane showing stenosis of the middle cerebral artery in its M2 segment, with evident involvement of the dorsal and ventral branches, and associated multiple collateral pial and leptomeningeal temporal arteries.
The prevalence of MMD in the Hispanic population is very low, making it a rare occurrence in this ethnic group [2, 3, 5, 6, 8]. Studies in other ethnicities, such as Indian populations, have shown different behaviors of MMD, such as a lower hemorrhagic presentation compared to the Japanese population [9]. This highlights the importance of reporting rare cases, such as the one presented here to better understand the disease's behavior across different ethnicities.
This case report provides a unique opportunity to review the clinical and imaging characteristics of MMD, as well as recent advances in its diagnosis and treatment. It represents excellent academic value to clinicians and postgraduate medical students, as it provides a comprehensive review of the patient's clinical presen- tation, diagnostic process, and treatment approach. Additionally, it discusses recent advances in the diagnosis and management of Moyamoya disease, thus improving understanding and knowledge of this rare condition.
2. CASE REPORT
A 41-year-old woman native to the Pacific coast of Mexico presented to the emergency department with a severe pulsating headache that spanned her entire head. This was accompanied by nausea, vomiting, and loss of alertness over a period of 15 days. On arrival at the emergency department, the patient had normal vital signs and no focal neurological deficits.
A plain head CT scan revealed interhemispheric subarachnoid hemorrhage with intraventricular extension into the occipital recess and the right atrium (Fig. 1A). Further imaging with CT and 3D angiographic reconstruc- tions showed significant stenosis of the middle cerebral artery in its M2 segment, along with multiple collateral arteries that extended throughout the cisterns, with numerous perforated branches. This imaging provided the classic appearance of a puff of smoke associated with Moyamoya disease (Fig. 1B-D).
To confirm these findings, the patient underwent digital subtraction angiography (DSA). The DSA further elucidated multiple collateral vessels dependent on the posterior cerebral arteries and the perforating thalamic arteries, strengthening the characteristic appearance of the puff of smoke. Furthermore, significant stenosis was identified in the M1 segment and the dorsal branch of the left middle cerebral artery (Fig. 2A-C).
Perfusion-weighted imaging (PWI) demonstrated normal relative cerebral blood flow, blood volume, and mean transit time across both hemispheres (Fig. 3A-B). Given these imaging findings and clinical presentation, the patient was diagnosed with Moyamoya disease.
Surgical intervention was considered necessary due to the severity of her condition. A primary revascularization attempt involving an end-to-end anastomosis of the branches of the superficial temporal artery (STA) with M4 branches of the right MCA was unsuccessful due to the poor condition of the recipient artery. Consequently, an indirect revascularization procedure, encephaloduroar teriosynangiosis, was performed. This procedure involved suturing the branches of the STA to the dura mater (Fig. 3C-D).
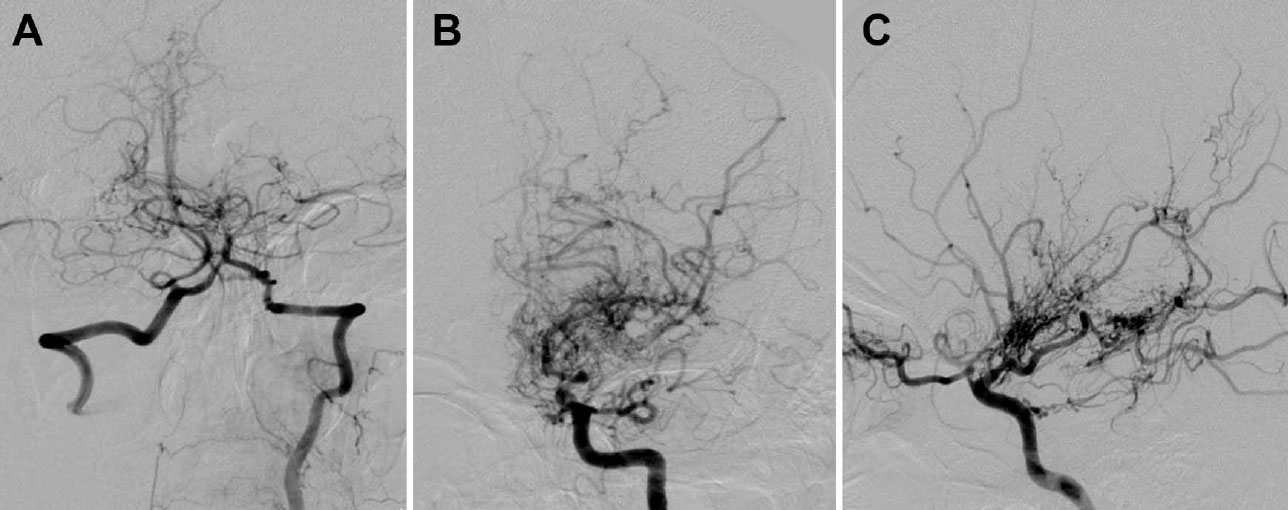
Digital subtraction angiography in the arterial phase. (A) Multiple collateral vessels dependent on the posterior cerebral arteries and the perforating thalamic arteries, with microaneurysms in the communicating artery of the left posterior. (B) Formation of abnormal net-shaped vessels that create the characteristic puff-of-smoke appearance. (C) Significant stenosis identified in segment M1 and the dorsal branch of the left middle cerebral artery.
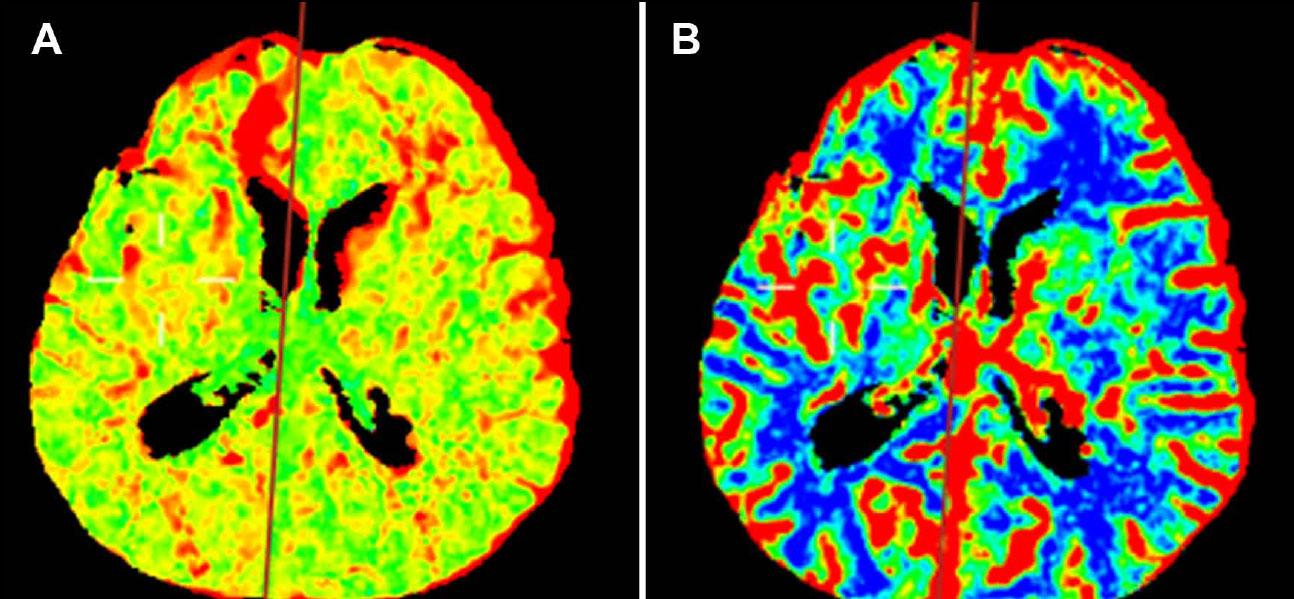
Perfusion-weighted image (PWI) color maps. (A) Mean transit time (MTT) map showing a symmetric distribution without evidence of acute ischemia. (B) Cerebral blood flow (CBF) map showing symmetric distribution. (C and D) After the indirect revascularization procedure, the reconstruction angiotomography of the maximum intensity projection (MIP) evidences a branch of the superficial temporal artery adjacent to the right superficial temporal-parietal artery (white arrows).
After surgery, the patient was transferred to the Recovery Unit for immediate postoperative care. She remained stable and continued her follow-up in the outpatient neurosurgery clinic. A detailed timeline of her diagnostic and treatment approach is provided in Table 1.
| Timeline | |
|---|---|
| 1 | Intense holocraneal pulsatile headache, emesis, and loss of consciousness experienced by the patient |
| 2 | Admission to the emergency department |
| 3 | A plain brain CT was performed, revealing subarachnoid hemorrhage. |
| 4 | DSA, 3D-CT, and CBF complete the diagnosis of MMD |
| 5 | The primary revascularization attempt failed due to poor recipient MCA condition. |
| 6 | Indirect revascularization (encephaloduroarteriosynangiosis) was successfully performed. |
| 7 | The patient continued her follow-up in the outpatient neurosurgery clinic. |
| Ischemic MMD | Hemorrhagic MMD [8] |
|---|---|
| TIA | Hemorrhagic stroke |
| Ischemic stroke | Headache from dilated transdural collaterals [8] |
| Seizures | - |
| Headache | - |
| Cognitive impairment | - |
|
Involuntary movements Chorea (most common) [2] Choreoathetosis Dyskinesia Dystonia Limb-shaking Epilepsia-partialis continua |
Haemorrhage from fragile collateral vessels [8]: Intraparenchymal Intraventricular Subarachnoid |
3. DISCUSSION
MMD presents with two primary types of symptoms: hemorrhage and ischemia [2, 3]. The patient, in this case, a 41-year-old woman, exhibited the predominant form of MMD in adults, which is intracranial hemorrhage. Disturbance of consciousness is the most common symptom in hemorrhagic MMD, which was observed in this patient [1, 2, 6]. Table 2 compares the clinical manifestations between hemorrhagic and ischemic presentations of MMD.
3.1. Ethnic Disparities in Moyamoya Disease
Moyamoya disease (MMD) is predominantly reported in East Asian populations, particularly Japan and Korea, where the incidence ranges from 0.35 to 0.94 per 100,000 individuals annually. In contrast, MMD is relatively rare in Hispanic populations, raising important questions about the genetic and environmental factors that contribute to this disparity [10].
3.2. Manifestation of Diseases
The clinical presentation of MMD can vary significantly among different ethnic groups. In East Asian patients, MMD often presents with ischemic symptoms in children and hemorrhagic symptoms in adults [1]. In contrast, the limited data on Hispanic patients suggest that they may exhibit a different spectrum of symptoms and disease progression. For example, a study of MMD in Hispanic patients found a lower incidence of hemorrhagic stroke compared to East Asian patients, which may indicate underlying differences in the pathophysiology of the disease [11].
3.3. Importance of Ethnic-specific Research
Addressing these ethnic disparities is crucial to developing customized diagnostic and therapeutic strategies. Understanding the genetic and environmental factors unique to different populations can help identify individuals at risk, improve early diagnosis, and inform the development of targeted treatments. Additionally, increasing awareness and education about MMD in under- represented populations can facilitate better clinical outcomes through early intervention and appropriate management [12].
3.4. Genetic Factors
The identification of the RNF213 gene as a major susceptibility factor for MMD in East Asian populations has significantly advanced our understanding of the genetic underpinnings of the disease. The RNF213 gene, located on chromosome 17q25, encodes an E3 ubiquitin-protein ligase involved in various cellular processes, including angiogenesis and vascular development [8]. Mutations in RNF213, particularly the variant p.R4810K, are strongly associated with MMD in East Asians but are less frequently observed in other ethnic groups, including Hispanics [6]. This genetic variability suggests that while RNF213 plays a crucial role in the pathogenesis of MMD, other genetic factors may contribute to disease susceptibility in different populations [13].
3.5. Environmental Factors
Environmental factors, such as geographic location, lifestyle, and exposure to certain risk factors, can also influence the prevalence and manifestation of MMD. For example, higher levels of air pollution, eating habits, and the prevalence of other vascular risk factors, such as hypertension and diabetes, in different regions could affect the development and progression of MMD. However, specific environmental factors that contribute to MMD in Hispanic populations have not been well studied, and further research is necessary to elucidate these factors [14].
Exploring the genetic and environmental factors that contribute to the rarity of MMD in Hispanic populations provides valuable information on the pathophysiology of the disease and highlights the need for ethnic-specific research. Such efforts are essential for developing comprehensive and effective strategies to diagnose, treat, and ultimately prevent MMD in diverse populations [15].
3.6. Diagnosis
The diagnostic evaluation of MMD is based on both anatomical and functional imaging techniques. Cerebral digital subtraction angiography (DSA) remains the gold standard for diagnosing MMD, providing detailed visualization of stenoocclusive lesions and the character- istic collateral vessel formation of the circle of Willis. In this case, DSA confirmed the diagnosis by demonstrating multiple collateral vessels dependent on the posterior cerebral arteries and the perforating thalamic arteries, characteristic of the appearance of a puff of smoke (Fig. 2A-C).
| Suzuki CTA Score | MRA Score Proposed by Houkin et al. | ||
|---|---|---|---|
| Stage | Imaging Findings | MRA Findings | MRA Score |
| 1 | Narrowing begins at the ICA bifurcation and at the fork of the carotid artery. |
ICA Normal Stenosis of C1 Discontinuity of the invisible C1 signal Invisible |
0 1 2 3 |
| 2 | Presence of moyamoya collaterals around the narrowed vessels |
MCA Normal Stenosis of M1 Discontinuity of the C1 signal Invisible |
0 1 2 3 |
| 3 | Aggravation of moyamoya collaterals around the narrowed vessels |
ACA Normal A2 and its distal part A2 and its distal signal decrease or loss Invisible |
0 1 2 |
| 4 | Worsening of the narrowed vessels, and moyamoya collaterals begin to fade. |
PCA Normal P2 and its distal region P2 and its distal signal decrease or loss Invisible |
0 1 2 |
| 5 | Occlusion of associated large vessels and more obvious reduction of surrounding moyamoya changes | Total score | 0-10 |
| 6 | The disappearance of moyamoya collaterals and vessels of the ICA system. The territories of the ICA are supplied from the external carotid artery. |
MRA findings 0-1 2-4 5-7 8-10 |
MRA score 1 2 3 4 |
Other imaging modalities, such as magnetic resonance imaging (MRI) and computed tomography (CT), are also crucial in the initial assessment of the extent of the disease, its impact on cerebral perfusion, and follow-up of patients with MMD [1-3].
The patient’s initial plain CT scan revealed interhemispheric subarachnoid hemorrhage with intra- ventricular extension, prompting further investigation with angiographic CT and 3D reconstructions (Fig. 1A-D). Perfusion-weighted imaging (PWI) provided additional information on cerebral blood flow, blood volume, and mean transit time, all of which were normal in this patient, suggesting preserved cerebral perfusion (Fig. 3A, B).
Recent advances in imaging modalities, such as perfusion MRI, xenon-enhanced CT (Xe-CT), positron emission tomography (PET), and single photon emission CT (SPECT), offer enhanced quantification of cerebro- vascular reserve capacity (CVR) and cerebral blood flow (CBF). These techniques can be integral in treatment planning and prognosis [1-3, 5, 6]. Furthermore, the Suzuki classification system and newer scoring systems, such as those proposed by Houkin et al., provide valuable frameworks for assessing the severity of MMD (Table 3) [1, 2].
3.7. Treatment
Treatment of MMD involves medical and surgical approaches. Medical treatment focuses on controlling symptoms and preventing complications. Antiplatelet agents, such as aspirin, are commonly used to reduce the risk of ischemic events. In cases of severe ischemia or recurrent TIA, surgical revascularization procedures remain the cornerstone of MMD management, aimed at restoring cerebral perfusion and preventing recurrent ischemic strokes. These procedures aim to improve cerebral blood flow by bypassing occluded vessels.
Recent studies have demonstrated the potential benefits of acetazolamide, a carbonic anhydrase inhibitor, in the treatment of Moyamoya disease [16]. Acetazolamide acts as a vasodilatory agent, producing a long-lasting increase in cerebral blood flow (CBF). This medication is used to assess cerebrovascular reserve (CVR), which is crucial in assessing the brain's capacity to increase blood flow under varying physiological conditions [17]. Acetazolamide administration has been shown to provide significant vasodilatory responses, improve CBF, and help identify regions with impaired vascular reactivity [18, 19]. Furthermore, an increase in CBF induced by acetazolamide can help better characterize the hemo- dynamic state of patients with Moyamoya disease, contributing to more informed treatment planning [17, 20].
The two main surgical approaches are direct and indirect revascularization. Direct techniques, such as the superficial temporal artery to the middle cerebral artery bypass (STA-MCA), directly connect the extracranial and intracranial arteries, providing immediate improvement in blood flow. Indirect techniques, such as encephaloduroar- teriosynangiosis, promote the formation of new collateral vessels with time. In this case, the patient underwent an unsuccessful primary revascularization attempt with an STA-MCA bypass due to a poor recipient artery condition, which required an indirect revascularization procedure (encephaloduroarteriosynangiosis) (Fig. 3C, D) [4].
Although direct revascularization is often preferred, indirect methods are particularly useful when direct anastomosis is not feasible. The 2015 Japanese guidelines for managing stroke recommend surgical revascularization for the ischemic type of MMD, although its efficacy in hemorrhagic MMD remains controversial [2]. Surgical revascularization in MMD can be categorized into three approaches: direct, indirect, and combined bypass procedures. Direct bypass techniques, such as STA-MCA, provide an immediate improvement in blood flow by directly connecting the external carotid artery system to the intracranial circulation. Indirect methods, including encephaloduroarteriosynangiosis, depend on angiogenesis to form new blood vessels over time. Combined approaches use direct and indirect techniques to maximize revascularization [15].
A more comprehensive analysis of treatment algorithms and their implications for patient management is warranted. The choice of surgical technique is influenced by factors, such as the age of the patient, the severity of the symptoms, and the presence of comorbid conditions. Studies have shown that combined revascula- rization techniques may offer better results in terms of long-term cerebral perfusion and reduced stroke recurrence [20, 21]. However, the optimal approach must be individualized based on the specific clinical scenario of the patient and the surgeon's expertise [14, 22].
Future research should focus on refining these treatment algorithms and exploring novel therapeutic targets to improve patient outcomes. Integration of advanced imaging modalities and computational methods, such as automated segmentation and predictive modeling, could further improve surgical planning and postoperative evaluation [12, 23]. Collaborative efforts among neurologists, neurosurgeons, and radiologists are essential to optimize treatment strategies and improve the quality of life of patients with Moyamoya disease.
3.8. Differential Diagnosis
The differential diagnosis of Moyamoya disease (MMD) includes several conditions that can present with similar clinical and radiographic features. It is crucial to distinguish MMD from these conditions to ensure appropriate management. Atherosclerosis, characterized by plaque buildup within the arteries, must be considered, especially in older patients with risk factors such as smoking, high cholesterol, and hypertension [10, 11]. Autoimmune diseases, which can also cause vascular changes, typically present with systemic symptoms and specific markers that are absent in MMD [24]. Meningitis, an inflammatory condition that affects the meninges, can mimic MMD but is generally accompanied by fever and signs of infection [22]. Brain tumors, particularly those that affect the basal ganglia, can present with headaches and neurological deficits similar to MMD, but imaging studies can differentiate these conditions based on the presence of a mass lesion [13].
Patients with Down syndrome are at increased risk of Moyamoya syndrome, a variant of MMD associated with other genetic conditions [6, 25]. Neurofibromatosis type 1, another genetic disorder, can cause vascular abnor- malities, but the pattern is different from that seen in MMD [21, 25]. Traumatic brain injury (TBI) and cranial irradiation can cause vascular changes and mimic MMD; however, a detailed history and imaging can help differentiate these conditions based on the presence of trauma or radiation exposure [11, 25].
3.9. Future Research Directions
MMD remains a challenging condition, and many aspects of its pathophysiology and treatment have not yet been fully understood. Addressing these knowledge gaps is crucial to improving patient outcomes. This section outlines several areas where future research is needed to improve our understanding and treatment of MMD.
3.9.1. Advanced Imaging Techniques
Future research should focus on the development and refinement of advanced imaging techniques to better understand cerebral hemodynamics and vascular changes in MMD. Techniques, such as quantitative color-coded parametric DSA (QDSA), dynamic 3D and 4D CTA, and ultrahigh field MRI (7.0-T) hold promise to provide more detailed and accurate assessments of cerebral blood flow (CBF) and cerebrovascular reserve (CVR) [10, 26]. These advanced imaging modalities can help identify subtle hemodynamic changes and, more precisely, monitor the effectiveness of surgical interventions.
3.9.2. Genetic and Molecular Mechanisms
Understanding the genetic and molecular mechanisms underlying MMD is critical. The role of the RNF213 gene, particularly the variant p.R4810K, has been well documented in East Asian populations, but its prevalence and impact in other ethnic groups, including Hispanics, remain unclear [6, 8]. Investigating other potential genetic markers and molecular pathways involved in MMD can provide insight into its etiology and lead to the development of targeted therapies.
3.9.3. Longitudinal Studies and Multicenter Trials
Large-scale multicenter longitudinal studies are needed to evaluate the long-term results of various treatment modalities in patients with MMD. Such studies can help determine the most effective surgical and medical management strategies and identify factors that influence the prognosis [15]. Furthermore, standardized protocols for follow-up imaging and clinical evaluations should be established to facilitate comparative studies and meta-analyses.
3.9.4. Differences in Pediatric and Adult Age
Research should also address differences in the presentation and progression of MMD between pediatric and adult patients. Although surgical revascularization techniques have been well studied in children, the efficacy and safety of these procedures in adults, particularly those with hemorrhagic MMD, need further investigation [11, 27]. Understanding these differences can lead to age-specific treatment guidelines and improved patient care.
3.9.5. Nonsurgical Interventions
Exploring nonsurgical interventions, such as pharmacological treatments and lifestyle modifications is another important area of research. The potential benefits of drugs, such as acetazolamide, which acts as a vasodilatory agent to improve cerebral blood flow (CBF), should be further studied to determine their role in the management of MMD [12]. Furthermore, lifestyle factors that can influence disease progression and patient outcomes must be investigated. For example, dietary habits, physical activity, and control of vascular risk factors, such as hypertension and diabetes can play an important role in the management of MMD.
3.9.6. Cognitive and Psychological Results
Future research should also examine the cognitive and psychological outcomes of MMD patients, both before and after surgery. The impact of improved cerebral perfusion on cognitive function and quality of life is an important area that remains underexplored [28]. Studies using functional imaging techniques, such as BOLD-fMRI, can help elucidate the relationship between cerebral hemodynamics and cognitive function. Understanding these results can guide comprehensive care strategies that address not only the physical but also the mental health needs of MMD patients.
3.9.7. Genetic Studies
Recent advances in understanding MMD have led to new insights into its pathophysiology and potential treatment strategies. Genetic studies have identified several potential susceptibility genes associated with MMD, suggesting a hereditary component of the disease. Furthermore, research on the molecular mechanisms underlying vascular proliferation and remodeling in MMD has opened new avenues for targeted therapies [5, 6, 8].
3.9.8. Advanced Imaging Techniques and Computational Methods
Future research in MMD could benefit from advanced imaging techniques and computational methods. Automated segmentation of MRI images using 2D U-Net predictions in axial, sagittal, and coronal views could improve diagnostic accuracy and efficiency [29]. Further- more, experimental diagnostic systems that incorporate fuzzy logic and neuro-fuzzy technology could assess treatment effectiveness in a more comprehensive way [30]. Deep neural networks and convolutional neural network (CNN) models with stratified k-fold cross-validation could also improve predictive modeling and treatment planning [31, 32].
Although some magnetic resonance imaging techniques have been known for more than a decade, their application to quantify the values of the apparent diffusion coefficient (ADC) in the penumbra, infarct, and normal regions of the brain of MMD could significantly help prevent stroke [33]. Understanding the diagnostic perfor- mance of perfusion-weighted imaging parameters (PWI), such as time to peak (TTP), mean transit time (MTT), relative cerebral blood volume (rCBV), and relative cerebral blood flow (rCBF), also improves the evaluation of ischemic stroke [34, 35]. These parameters provide additional biomarkers for the diagnosis and follow-up of patients with MMD, thus improving clinical outcomes through more precise monitoring and customized interventions.
CONCLUSION
This case report highlights the unique presentation and treatment of MMD in a 41-year-old Hispanic woman, emphasizing the importance of recognizing this rare condition in diverse populations. The clinical presentation of the patient and imaging findings underscore the need for advanced diagnostic techniques, such as perfusion-weighted imaging (PWI) and quantitative color-coded parametric DSA, to enhance diagnostic precision and treatment planning. The successful application of indirect revascularization through encephaloduroarteriosynan- giosis demonstrates the efficacy of surgical interventions in the treatment of MMD.
Exploring genetic factors, particularly the role of the RNF213 gene, alongside environmental influences, can provide valuable information on the pathophysiology of the disease and contribute to customized therapeutic strategies. Addressing ethnic disparities in MMD is crucial to improving early diagnosis and patient outcomes. Future research should focus on refining treatment algorithms, investigating nonsurgical interventions, and examining cognitive and psychological outcomes to further improve patient care. This case underscores the need for a comprehensive and multidisciplinary approach to the treatment of MMD and the importance of continuing research to bridge knowledge gaps and improve the lives of those affected by this complex disease.
AUTHORS’ CONTRIBUTIONS
It is hereby acknowledged that all authors have accepted responsibility for the manuscript's content and consented to its submission. They have meticulously reviewed all results and unanimously approved the final version of the manuscript.
LIST OF ABBREVIATIONS
| ACA | = Anterior cerebral artery |
| ADC | = Apparent diffusion coefficient |
| BOLDfMRI | = Blood Oxygen Level Dependent Functional Magnetic Resonance Imaging |
| C1 | = Cervical vertebra 1 |
| C2 | = Cervical vertebra 2 |
| C3 | = Cervical vertebra 3 |
| CBF | = Cerebral blood flow |
| CFB | = Cerebrovascular flow |
| CNS | = Central nervous system |
| CRP | = Creactive protein |
| CTA | = Computed tomography angiography |
| CVR | = Cerebrovascular reserve capacity |
| DSA | = Digital subtraction angiography |
| ICA | = Internal carotid artery |
| MCA | = Middle cerebral artery |
| MMD | = Moyamoya disease |
| MRA | = Magnetic resonance angiography |
| MRI | = Magnetic resonance imaging |
| MTT | = Mean transit time |
| PCA | = Posterior cerebral artery |
| PET | = Positron emission tomography |
| PWI | = Perfusionweighted imaging |
| QDSA | = Quantitative colorcoded parametric digital subtraction angiography |
| rCBF | = Relative cerebral blood flow |
| rCBV | = Relative cerebral blood volume |
| SPECT | = Single photon emission computed tomography |
| STA | = Superficial temporal artery |
| STAMCA | = Superficial temporal artery to the middle cerebral artery |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The reported case adheres to the ethics code of the Human Experimentation Committee and the official Mexican Norm NOM-012-SSA3-2012.
HUMAN AND ANIMAL RIGHTS
All human research procedures followed were in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013.
CONSENT FOR PUBLICATION
An informed consent form was obtained for the present research work. All images included (CT, angiography, perfusion maps) in the manuscript were anonymized. Therefore, the submission did not include information that might identify the patient. In addition, all diagnostic studies were performed as part of routine care.
AVAILABILITY OF DATA AND MATERIALS
The medical information related to the case report was sourced from the medical records of the Instituto Nacional de Neurología y Neurocirugía in Mexico City.


