All published articles of this journal are available on ScienceDirect.
Differential Effects in the Subsystems of the Salience Network in Schizophrenia
Abstract
Background
Recent studies have identified links between schizophrenia and abnormalities in the brain's salience network, a crucial system with primary hubs in the anterior insula and anterior cingulate cortex. This network is divided into two subsystems: the dorsal salience network, which processes sensory information and allocates attention to self-generated or external sensory stimuli, and the ventral salience network, associated with processing the emotional valence of stimuli sensations.
Methods
This pilot study analyzed the resting state functional magnetic resonance imaging (fMRI) data from 14 schizophrenia patients and 16 healthy controls. We focused on the functional connectivity within the salience network's dorsal and ventral subsystems, particularly between the dorsal anterior insula and frontoparietal areas, and the ventral anterior insula and the subgenual anterior cingulate cortex.
Results
The analysis revealed that schizophrenia patients displayed weaker connectivity within the dorsal salience network, notably between the dorsal anterior insula and frontoparietal areas. In contrast, these patients demonstrated increased connectivity within the ventral salience system, especially between the ventral anterior insula and the subgenual anterior cingulate cortex.
Conclusion
Our findings highlight that disruptions in the salience network in schizophrenia vary depending on the type of information being processed. This variance underscores the complexity of the disorder and the specific challenges it poses to the brain's ability to process and prioritize information.
1. INTRODUCTION
One of the prominent hypotheses for the pathophysiology of schizophrenia is that of aberrant salience linked to the abnormal signalling in the dopaminergic pathways of the brain [1]. According to this hypothesis, the brain uses internal models to integrate incoming sensory information. Sensory information is considered salient when it violates the predictive internal model of the world. Persistent mismatches between predicted and actual stimuli result in adaptive changes in the internal brain models of the world, and this process is tuned by the dopaminergic signalling pathways of the brain [2]. The misattribution of salience from external stimuli to self-generated actions leads to aberrant salience that manifests as delusional ideas and hallucinations [2].
Recent advances in functional brain imaging and, more specifically, the use of connectivity analysis of the resting state functional brain imaging (rsfMRI) signals have allowed researchers to identify a salience functional network in the human brain. The salience network comprises the anterior insula (AI) and dorsal anterior cingulate cortex (dACC), as well as the anterior prefrontal cortex (APFC), the supramarginal gyrus (SMG), the striatum/basal ganglion, the thalamus and the cerebellum [3-5]. This network was shown to be involved in integrating internal and external salient information and was activated across a wide range of cognitive and affective tasks [6, 7]. In addition, the salience network was found to control the interaction between the default mode network and the central executive network. The salience network controls the engagement of the central executive network and the disengagement of the default mode network to mediate the cognitive control process when a salient external event is detected [3].
A series of studies have documented reduced connectivity of the salience network in patients with schizophrenia [8, 9]. In a meta-analysis of studies using resting state fMRI in patients with schizophrenia and healthy controls, the dysfunction of the salience network and the imbalanced communication of this network with other functional networks were hypothesised to underlie the core difficulty of patients to differentiate self-representation and environmental salience processing as suggested by the aberrant salience hypothesis of schizophrenia [10]. More recently, the dysfunction of the salience network has been documented in patients with first episode of psychosis [11, 12], antipsychotic naïve first episode of psychosis [13] and even ultra-high risk for developing psychosis individuals [14].
Recent research proposes a dual-subsystem structure of the salience network, centered on the dorsal and ventral anterior insula [15, 16]. The ventral subsystem is thought to relate to salience processing via affect, by contributing to the generation and interpretation of bodily feelings so that an individual becomes aware of the value of information in the context of present needs or goals. Enhanced connectivity in this system has been linked with increased emotional experiences. On the other hand, the dorsal subsystem regulates goal-driven attention, enhancing the activation of task-relevant and suppressing task-irrelevant networks to process whatever information is most relevant to the goal at hand. Enhanced connectivity in this system correlates with improved performance in attention tasks [16]. Previous schizophrenia research hasn't delved into the differences between these subsystems of the salience network.
In this pilot study we used functional connectivity analysis of the rsfMRI to evaluate the intrinsic connectivity of both salience subsystems in schizophrenia patients and healthy controls. Based on the aberrant salience hypothesis of schizophrenia, we predicted dysfunction of the salience network in patients compared to controls. We also predicted a differential pattern of connectivity differences in the subsystems of the salience network in these patients.
2. METHODS
2.1. Participants
14 patients diagnosed with schizophrenia, according to the Diagnostic and Statistical Manual of Mental Disorders, Revised Fourth Edition (DSM-IV), were enrolled in the study, comprising 9 men and 5 women. The mean duration of illness among the patients was 7 years, with a mean treatment duration of 5.4 years. The average age of the subjects was 31 years old. Each patient received treatment with one atypical antipsychotic medication, such as olanzapine, aripiprazole, amisulpride, or risperidone, administered at therapeutic doses. Additionally, 16 healthy control participants, consisting of 9 men and 7 women, were included in the study. All subjects were right-handed, and control participants were matched with patients based on age, sex, race, and handedness criteria. While all patients were receiving psychotropic medication, none of the control participants were taking medication that affected the CNS. Each participant provided written informed consent, and the study protocol was approved by the ethics committee of Eginition University Hospital.
2.2. Magnetic Resonance Imaging (MRI) Procedures
Image acquisition was performed using a 3T Philips manufactured Achieva TX scanner located at Eginition Hospital, equipped with an 8-channel phased-array head coil. To reduce head movement, head restraints like a pillow and foam padding were employed. Ear plugs were used to minimize noise disturbance. All participants were scanned with the same brain imaging protocol, including a structural T1-weighted high resolution 3D T1-TFE sequence to [TR/TE/flip angle = 9.9 ms/ 7.7 ms/7°, and a resolution of 1.0 mm x 1.0mm x 1.0mm] and an echo-planar imaging gradient echo sequence [TR/TE/flip angle = 2000 ms/ 30 ms/ 900 and a resolution of 3.0 mm x 3.0mm x 3.0mm, 264 dynamic scans] for resting-state functional MRI.
During these resting-state fMRI sessions, participants were instructed to keep their eyes open without fixating on anything and to maintain stillness.
2.3. Data Preprocessing
The resting state fMRI data underwent preprocessing according to the standardised resting state functional connectivity MRI (rs-fcMRI) protocols [17-19]. Firstly, the first four dynamic scans were excluded and then we performed:
- Slice time correction (SPM2, Wellcome Department of Cognitive Neurology, London, UK).
- Motion correction employing rigid-body transformation addressing three translations and rotations (conducted at FMRIB, Oxford, UK).
- Spatial resampling to 2mm isotropic voxels and normalization to the Montreal Neurological Institute (MNI) atlas space.
- Spatial smoothing using a 6mm full-width-at-half-maximum (FWHM) Gaussian kernel.
- Temporal filtering to exclude frequencies> 0.08Hz.
- Finally, potential sources of non-specific variance and their time-based derivatives were removed from the dataset via linear regression (this includes six parameters from rigid-body motion correction, averaged signals from the entire brain, deep white matter, and the ventricles). The remaining BOLD time series were preserved for subsequent functional connectivity examinations.
2.4. Resting State FMRI Analysis
To assess the intrinsic functional connectivity strength within the dorsal and ventral salience subsystems, we employed seed-based rs-fcMRI methodology. Using a hypothesis-driven method, we constructed spherical regions of interest (ROIs) with a 4-mm radius centered on the dorsal and ventral anterior insula areas.
Functional connectivity within the dorsal salience subsystem was determined estimating the Pearson's correlation coefficients between the average time progression of the Blood-Oxygen-Level-Dependent (BOLD) signal from the dorsal anterior insula (dAI) (with coordinates in MNI: +36, 21, 1 for the right dAI) and each brain voxel, on an individual basis. These coefficients were then converted to z-scores through a Fisher transformation, consistent with the approach in previous research [16]. Similarly, for the ventral salience subsystem, we employed Fisher’s r-to-z correlation metrics between the average time progression of the BOLD signal from the ventral anterior insula (vAI) (coordinates for the right vAI: + 28, 17, -15).
We applied two-tailed t-tests to determine differences between the groups based on the averaged z-maps. We reported outcomes that withstood the family-wise error correction for multiple evaluations, using a global false discovery rate set at p < 0.05.
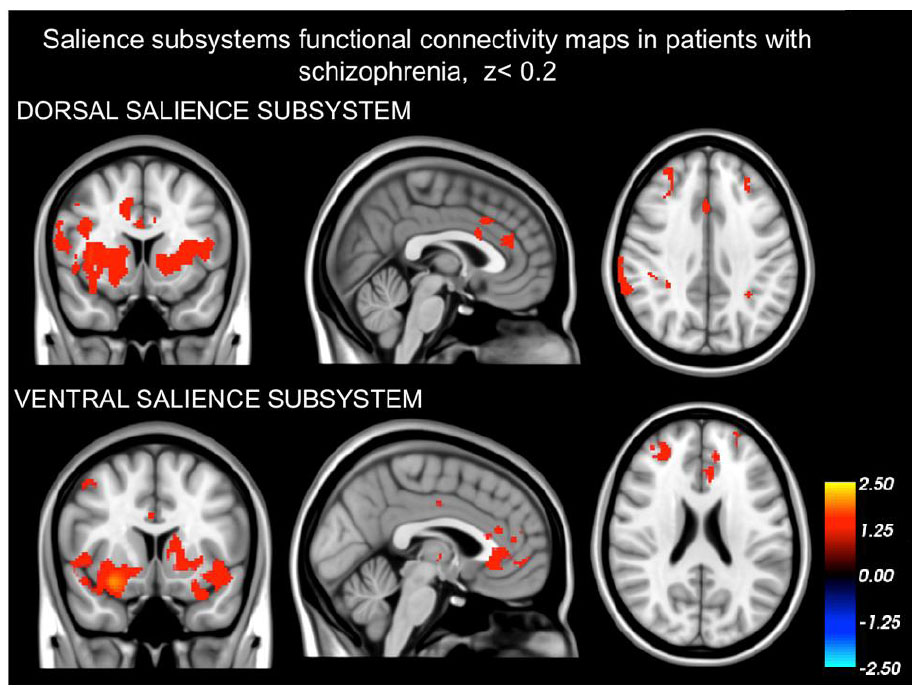
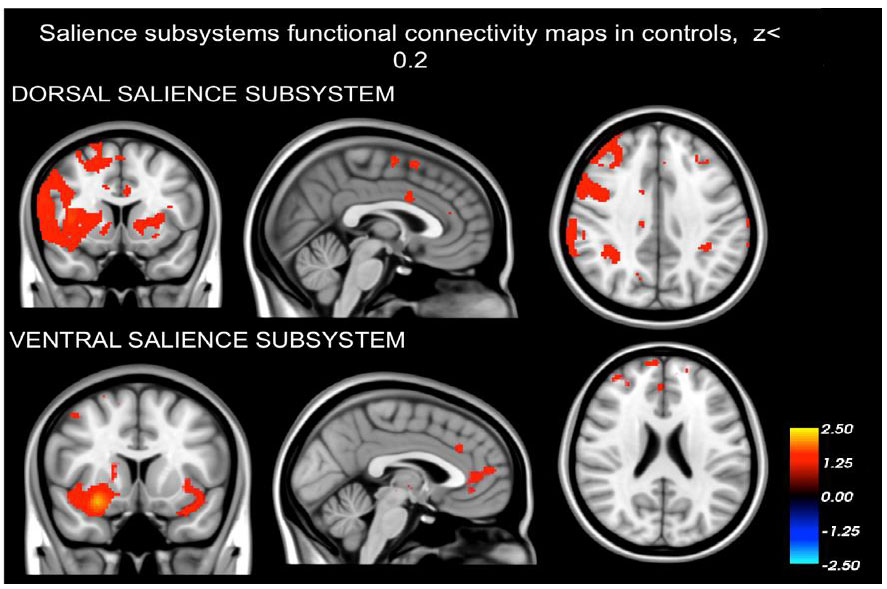
3. RESULTS
3.1. Dissociation of the Dorsal and Ventral Salience Subsystems
Our findings demonstrating that the networks identified in both schizophrenia patients (as depicted in Fig. 1) and healthy controls (Fig. 2) are distinct from one another. The intrinsic BOLD signal from the right dAI showed correlation with signals in the dorsal anterior cingulate cortices (dACC), mid cingulate cortex (MCC), regions in the frontal cortex situated in the precentral and postcentral gyrus, the parietal cortex (specifically the supramarginal gyrus), and areas of the insula (including dAI, bilateral vAI, and mid to posterior insula sections). On the other hand, the intrinsic BOLD signal rooted in the right vAI was in sync with signals in the perigenual ACC (pgACC), subgenual ACC (sgACC), orbitofrontal cortex, amygdala, and frontoinsula.
3.2. Group differences in the connectivity within the dorsal and ventral salience subsystems
Our findings revealed that, compared to healthy controls, individuals diagnosed with schizophrenia exhibited noticeably diminished functional connectivity in the dAI. Within the dorsal salience subsystem, these patients displayed reduced connectivity between the dAI and frontoparietal regions i.e. paracingulate segment of the midcingulate cortex, middle frontal gyrus, precentral gyrus and supplementary motor area (p<0.05, FWE corrected) (Figs. 2-4).
4. DISCUSSION
Our analysis confirmed the presence of distinct dorsal and ventral subsystems of the salience network in both healthy controls and patients with schizophrenia. Confirming our hypothesis, patients with schizophrenia exhibited differences in the functional connectivity patterns of the salience sub-networks when compared to healthy controls. Specifically, functional connectivity in the dorsal salience network was reduced, while functional connectivity in the ventral salience network was increased in patients compared to healthy controls.
The dorsal network encompassed regions such as the midcingulate, paracingulate, and frontal areas. On the other hand, the ventral network involved the subgenual cingulate, amygdala, and orbitofrontal cortex. Both in patients and healthy controls, these distinct salience networks align with findings from prior research on insula connectivity during rest [16]. Such results further bolster the evidence pointing to separate subsystems within the salience network.
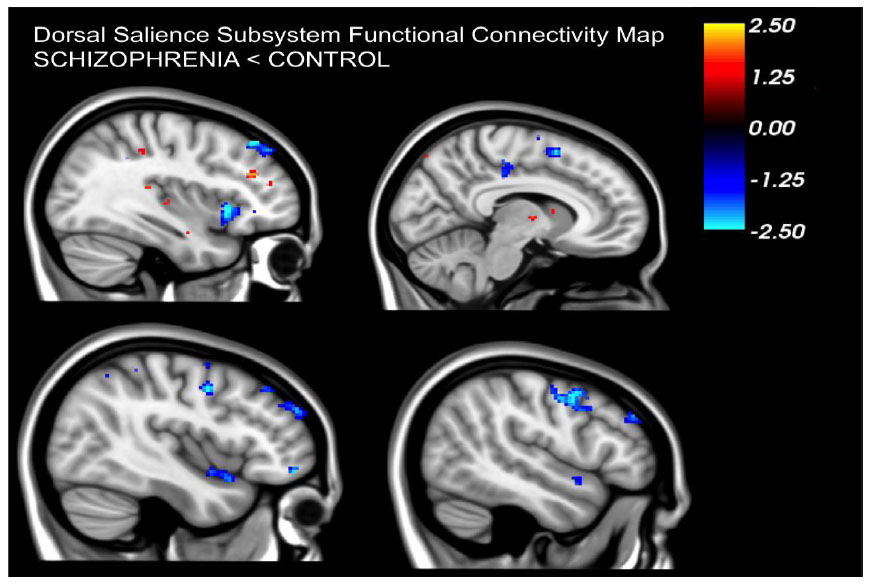
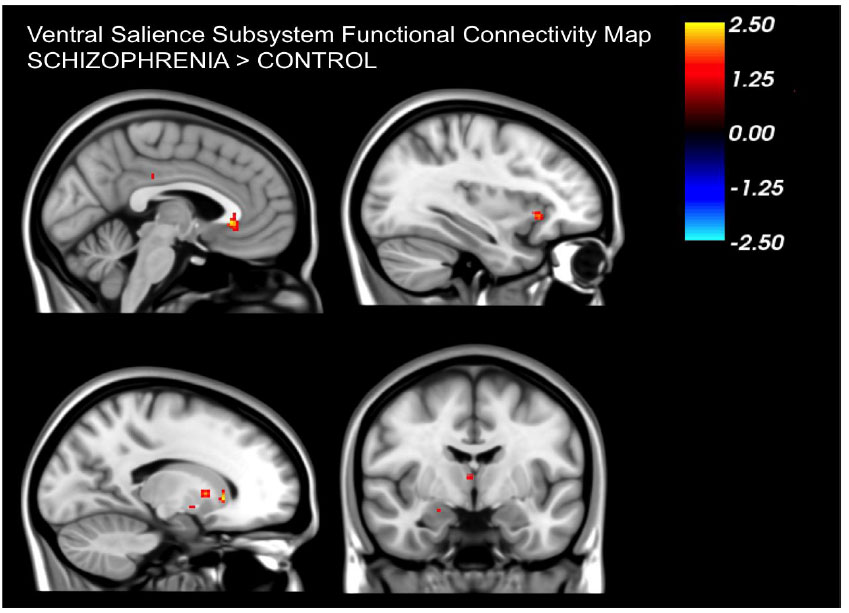
Patients displayed a noticeable decrease in resting connectivity within the dorsal salience network when compared to healthy controls. Past studies have linked this network's connectivity to attention-related tasks [16]. Reduced connectivity in the dorsal salience network could be directly linked to the salience aberrance hypothesis in schizophrenia suggesting that patients have difficulty in processing salient external stimuli and modulating attention between internal and external cues. Moreover, this salience network oversees the balance between default mode and task-positive networks [20]. Reduced connectivity in the dorsal salience network in patients might also be linked to the observed imbalance in these networks that has been reported in patients with schizophrenia [21-26].
In contrast to the reduced connectivity in the dorsal salience network we observed that the ventral salience network showed increased connectivity in patients. This network's connectivity has been associated with arousal levels, pointing to its role in determining the emotional importance of events [16]. The increased connectivity suggests that patients might perceive events as more emotionally significant. This also aligns with the aberrance salience hypothesis in schizophrenia in the sense that patients attribute increased salience to normal emotional reactions leading to the generation of symptoms such as delusions.
In summary, changes in connectivity in both the dorsal and ventral salience networks might be related to different symptoms in schizophrenia as predicted by the aberrant salience hypothesis. The decreased connectivity in the dorsal network results in attention and executive function deficits, potentially leading to hallucinations. Meanwhile, increased connectivity in the ventral network might cause disordered emotional processing and the formation of delusions.
It is important to acknowledge that while our findings provide valuable insights, they are derived from a relatively small sample size, which may constrain the generalizability of our results. The small sample size could potentially obscure nuances in the data and limit the extent to which our findings can be extrapolated to broader populations. Therefore, future investigations with larger samples are warranted to corroborate and broaden the scope of our findings.
CONCLUSION
This study has made significant contributions to understanding the differential effects within the salience network's subsystems in individuals with schizophrenia. Our results demonstrate distinct patterns of connectivity: reduced functional connectivity within the dorsal salience network and increased connectivity within the ventral salience network in schizophrenia patients compared to healthy controls. These findings align with and expand upon the aberrant salience hypothesis of schizophrenia, suggesting specific neural mechanisms behind the disorder's complex symptomatology. Reduced connectivity in the dorsal network could underlie difficulties in attention and executive function, potentially contributing to psychotic symptoms such as hallucinations. Conversely, the heightened connectivity in the ventral network may exacerbate emotional processing, leading to symptoms like delusions.
Our study underscores the importance of considering the salience network's role in schizophrenia, particularly how its dysregulation can impact the processing of sensory and emotional stimuli. Given the small sample size and exploratory nature of our study, further research with larger cohorts is essential to validate these findings. Future studies should also investigate the relationship between altered salience network connectivity and specific schizophrenia symptoms, potentially offering new targets for therapeutic interventions. Additionally, examining task-related brain activity in conjunction with resting-state connectivity could provide deeper insights into the functional implications of these neural alterations.
The exploration of the salience network in schizophrenia opens new avenues for understanding the neural underpinnings of the disorder and developing targeted treatments. By elucidating the distinct roles of the dorsal and ventral subsystems, our study contributes to a more nuanced view of schizophrenia's complex neurobiology.
LIST OF ABBREVIATIONS
| fMRI | = functional magnetic resonance imaging |
| dACC | = dorsal anterior cingulate cortex |
| AI | = Anterior insula |
| SMG | = Supramarginal gyrus |
| DSM-IV | = Diagnostic and Statistical Manual of Mental Disorders, Revised Fourth Edition |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study protocol was approved by the ethics committee of Eginition University Hospital in Athens, Greece (ADA B11Ψ46Ψ8Ν2-ΨΦ9 Athens 29/5/2014/295).
HUMAN AND ANIMAL RIGHTS
No animals were used in this research. All procedures performed in studies involving human participants were in accordance with the ethical standards of institutional and/or research committee and with the 1975 Declaration of Helsinki, as revised in 2013.
CONSENT FOR PUBLICATION
All participants signed a written informed consent
STANDARDS OF REPORTING
STROBE guidelines were followed.
AVAILABILITY OF DATA AND MATERIALS
The data supporting the findings of the article is available in the Aiginiteio University Hospital Database and available upon request https://eginitio-en.uoa.gr reference number: B11?46?8?2-??9 Athens 29/5/2014/29.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


