All published articles of this journal are available on ScienceDirect.
Evaluation of the DTI-ALPS Index as a Biomarker of the Glymphatic System at 1.5T
Abstract
Introduction
The glymphatic system is a waste clearance pathway within the brain that relies on the flow of cerebrospinal fluid facilitated by astrocytes. It has been proposed that this glymphatic system can be observed using Diffusion Tensor Image Analysis along the Perivascular Space (DTI-ALPS). Yet, all observations have been made at 3.0T while most clinical scanners worldwide operate at 1.5T. The change in magnetic field strength is significant, as it affects signal-to-noise ratio, spatial resolution, and the minimum echo time achievable for the same diffusion weighting, which implies different diffusion times embedded in the observations. The question remains on the usefulness of this index at 1.5T for the observation of pathologies, particularly those related to glial cells. This study aimed to evaluate the usability of the DTI-ALPS index as a biomarker for the glymphatic system using 1.5T MRI, focusing on reproducibility among different users and its capacity to distinguish pathological values in glioma patients.
Materials and Methods
A retrospective study included 44 glioma patients and 10 healthy volunteers, with DTI sequences acquired using a 1.5T MRI scanner. Patients whose structural anatomy at the level of the lateral ventricle was significantly modified by the tumor were excluded. Reproducibility between sessions and different users was evaluated on 16 healthy subjects from a public dataset. The ALPS index was calculated based on diffusivity measurements in the projection and association fibers. Four neuroradiologists independently placed regions of interest for ALPS index calculation. Statistical analyses included Intraclass Correlation Coefficients (ICC) to assess inter-rater reliability and linear regression models to analyze the relationship between ALPS index values and patient characteristics.
Results
In the data from healthy subjects, the inter-rater reliability was low (ICC = 0.34), indicating high variability among users. A negative correlation between the ALPS index and age was observed. In glioma patients, the ALPS index showed significant differences between ipsilateral and contralateral hemispheres (1.46 ± 0.24 vs. 1.31 ± 0.22, respectively), with the contralateral side exhibiting values closer to those of healthy subjects (1.65 ± 0.20).
Discussion
The reproducibility of the DTI-ALPS index is significantly affected by user variability. Further research is needed to standardize ROI placement and improve image processing techniques to enhance the reliability of the ALPS index in clinical practice.
Conclusion
Being easily implemented, the DTI-ALPS index demonstrates some potential as a non-invasive biomarker for glymphatic system function, particularly in identifying pathological changes in glioma patients, considering evaluation in ipsi- and contralateral hemispheres.
1. INTRODUCTION
The glymphatic system is a brain-waste clearance pathway that depends on the astrocytes-mediated flow of cerebrospinal fluid, formally proposed approximately a decade ago [1]. It was shown that the passage of tracer molecules with the highest molecular weight from the arterial perivascular spaces to the interstitial space and from the interstitial space to the venous perivascular spaces depends, in both cases, on active astrocyte transport, specifically on the presence of aquaporin-4 channels in the feet of these glial cells. The glymphatic system has raised much interest, as it is involved in the transport of beta-amyloid protein, which is crucial in the pathophysiology of Alzheimer’s disease [1, 2]. Several studies in the literature show the impact of different pathologies on this clearance system such as multiple sclerosis [3], Parkinson's disease [4], epilepsy [5], idiopathic intracranial hypertension [6], hydrocephalus [7], traumatic brain injury [8], dementia [9], Alzheimer’s disease [10], and glioma, which are of particular interest because of the relevance of glial cells in the proposed clearance pathways [11-13]. Recent studies have shown that glymphatic flow increases by 95% during sleep, particularly during the slow-wave phase. Some factors, such as alcohol intake, exercise, depression, and anxiety, could also change its efficiency [14, 15].
There is no doubt about the interest in observing this system, yet one of the questions that arises is how to characterize it non-invasively [16]. Initially, the glymphatic system has been studied through experiments with tracers, especially in animals. Studies with tracers are one of the most effective methods for visualizing the body's in vivo mass transport systems, as they allow for the analysis of a substance and its interactions within the body without altering its original properties. Some of the proposed imaging methods are based on dynamic contrast-enhanced MRI with intravenous gadolinium injection [17], real-time assessments of tetramethylammonium diffusion and two-photon imaging in live mice [18], and the use of radioisotopes to track transport within the interstitial space [19]. The use of intrathecal contrast-enhanced glymphatic MRI is increasing, with an off-label indication for gadobutrol, and accumulating evidence shows its safety [20]. However, these are preliminary studies that evaluate its importance in providing diagnostic information about disturbed Cerebrospinal Fluid (CSF) homeostasis and glymphatic failure.
On the other hand, non-invasive assessments have been proposed, such as the use of Arterial Spin Labeling, in mouse brains [20] and patients [21]. However, the proposal that has generated considerable interest is the one by Taoka et al. [2], which utilizes Diffusion Tensor Image Analysis along the Perivascular Space (DTI-ALPS). The underlying idea is as follows. At the level of the body of the lateral ventricle, the medullary veins run perpendicular to the ventricular wall, and the perivascular space runs in the same direction as the medullary veins, which is the right-left direction (X-axis). In this area, the projection fibers run in the head-toe direction (Z-axis), mainly adjacent to the lateral ventricle, and the superior longitudinal fasciculi, which represent the association fibers, run in the antero-posterior direction (Y-axis), outside of the projection fibers. Consequently, the perivascular space runs perpendicular to both projection and association fibers in this area. Therefore, this conformation of the perivascular space and the main fibers allows for an analysis that is almost independent of diffusivity along the direction of the perivascular space, as the tracts of the main fibers do not run parallel to this direction. Thus, when there is a histological change along the right-left direction, it will affect projection and association fibers equally. Therefore, when such a change is observed in both fiber bundles, it is likely that at least a part of this change stems from the pathology affecting the perivascular space, and hence the glymphatic system. This proposal enables a non-invasive evaluation of the glymphatic system in virtually all MRI scanners, eliminating the need for tracer injection.
Many studies have evaluated the use of the ALPS index in patients with different conditions and pathologies, such as epilepsy [5], idiopathic normal pressure hydrocephalus [22], traumatic brain injury [8], older adults at risk of dementia [9], sleep disruption [23], Parkinson’s disease [24], elderlies [25], headaches [26, 27], juvenile myoclonic epilepsy [28], cerebral small vessel disease [22], cancer pain [29], and in spontaneous intracerebral hemorrhage [30], among other examples. Its ease of implementation is attracting considerable interest in the community. The number of studies published using DTI-ALPS doubled between 2022 and 2023, reaching nearly 50 studies in 2023, as observed in a PubMed search. Yet, to the best of our knowledge, all observations have been made at 3.0T while most clinical scanners worldwide operate at 1.5T. The change in magnetic field strength is not trivial, as it influences signal-to-noise ratio, spatial resolution, and the minimum echo time achievable for the same diffusion-weighting—ultimately resulting in different diffusion times inherent in the measurements. This study aimed to evaluate the usability of the Diffusion Tensor Image Analysis Along the Perivascular Space (DTI-ALPS) index as a biomarker for the glymphatic system using 1.5T MRI, focusing on reproducibility among different users and its capacity to distinguish pathological values in glioma patients.
2. METHODS
This single-center retrospective study included 44 patients (23 women, mean age of all patients 40.4 ± 13.8 years), from the local public hospital in Valparaiso (Chile), and the diagnosis was confirmed through histopathology. Inclusion criteria were to present a low- or high-grade supratentorial glioma and to require excision surgery using awake craniotomy, as indicated by the neuro-oncological committee. Written informed consent was obtained from participants, and the study was approved by local ethics committee (Servicio de Salud Valparaíso San Antonio, Chile CEC-SSVSA #72/2017). WHO grade 2 gliomas were considered Low-Grade Gliomas (LGG), and grade 3 and 4 gliomas were classified as High-Grade Gliomas (HGG). Patients whose tumors extended to both hemispheres were excluded, as a comparison between the ipsilateral and contralateral hemispheres would not have been feesible. Acquired sequence artifacts or patients whose structural anatomy at the level of the lateral ventricle was significantly modified by the tumor were excluded, as a precise positioning of the Regions Of Interest (ROI) needed for the ALPS estimation would not have been possible. To compare the results in patients with those in healthy subjects using the same acquisition protocol, images from 10 healthy volunteers were also acquired (3 women, with a mean age of 25.0 ± 4.0 years). Images were acquired using a 1.5T General Electric Signa HDxt scanner (Waukesha, USA), with an 8-channel head coil. The following sequences were obtained as routine protocols in glioma patients: DTI, axial T1-weighted imaging (WI), T2*-WI, diffusion-weighted images (DWI), 3D T1-WI, axial Fluid-Attenuated Inversion Recovery (FLAIR) images, and axial T2-WI. In healthy subjects, anatomical images were obtained with a T1-weighted fast spoiled gradient echo sequence (FSPGR), TE/TR of 1.9/6.1 ms, 256x256 matrix, 24 cm FOV, and 1.2 mm slice thickness, giving 3D T1-weighted images. The DTI acquisition parameters were TE/TR of 95/12000 ms, b-value of 1000 s/mm2, 32 non-collinear diffusion-weighted encoding directions; and spatial resolution of 1.0 x 1.0 x 3 mm3.
In addition to the local data, reproducibility was evaluated on a public dataset, including 16 healthy controls (7 women, 49.8 ± 18.4 years old at the moment of the first session), examined twice on the same 1.5T scanner, with an interval of 3.3 ± 1.0 years, ds001378 from openneuro.org [31]. The image acquisition parameters were TE/TR of 89/9394ms, 2 x 2 x 3 mm3 voxel size, 3 repetitions, b-value of 1000 s/mm2, and 15 non-collinear diffusion directions.
All DTI processing was undertaken using DSI Studio (v3.8.2022) (https://dsi-studio.labsolver.org/), including artifact correction due to Eddy currents and motion, and DTI reconstruction. Pre-processing methods to correct eddy current distortions and subject motion during diffusion MRI analysis integrated tools such as TOPUP and EDDY alongside proprietary algorithms of DSI Studio [32]. Eddy currents and motion artifacts are addressed through slice-to-volume registration and outlier replacement. Linear registration was applied, based on mutual information between b=0 images and diffusion-weighted images, ensuring proper alignment while automatically adjusting the b-matrix to preserve diffusion gradient orientations. ROI placement was performed following the methodology described in the seminal paper by Taoka et al. (2017), with additional attention to anatomical consistency across subjects. Specifically, two Regions Of Interest (ROIs) were placed in each hemisphere—one within the projection fibers and one within the association fibers. Color-coded Fractional Anisotropy (FA) maps were used to delineate the ROIs. The location of the anatomical slice was carefully defined in the axial plane, at the level of the superior portion of the lateral ventricles, using the presence of the corona radiata as the prominent anatomical landmark. This choice ensures a consistent location for identifying both projection and association fibers, as this is a region where these structures are easily distinguishable in diffusion-weighted imaging. The ROI definition was based on identifying areas with greater color intensity in the FA maps, suggesting greater directional coherence of the fibers. Priority was given to selecting regions with a homogeneous hue— blue for projection fibers and green for association fibers. The recommendation was to consider structural images as a complement to correct anatomical delimitation, thereby more accurately identifying deep vascular structures, such as the deep medullary veins, and areas of interest characterized by high intensity in FA maps that are oriented perpendicular to the body of the lateral ventricles. Each ROI was defined with a fixed volume of 48 mm3. While the size was standardized, the shape of the ROIs (square, rectangular, or other) was adjusted as needed to conform to local anatomy and avoid overlap with adjacent structures. This approach aimed to strike a balance between anatomical accuracy and reproducibility across subjects. Four reviewers with 5 to 20 years of experience in neuroradiology independently evaluated MRI files and defined ROI placement.
After estimating the diffusion tensor, the ALPS index was calculated using Eq. (1):
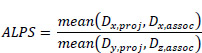 |
(1) |
where Dx,proj stands for the diffusivity on the X-axis (or right-left axis) in the ROI of the projection fibers, Dx,assoc for the diffusivity on the X-axis in the ROI of the association fibers, Dy,proj for the diffusivity in the Y-axis (or anterior-posterior axis) in the ROI of the projection fibers, and Dz,assoc for the mean diffusivity in the Z-axis (or superior-inferior axis) of the association fibers.
Statistical analysis was performed using Jamovi (v2.3.18) [https://www.jamovi.org]. For the entire study, statistical significance was defined with a p-value <0.05. The normality of the variables was verified in all cases using the Shapiro-Wilk method. Levene's test for the homogeneity of variances was used for all the t-tests. In the healthy subjects from the public dataset, the relationship between the ALPS index and sex was analyzed using Welch's t-test for independent samples, considering the inhomogeneity of variances with Levene's test. To analyze the correlation between the ALPS index and the age of the participants, linear regression and multiple linear regression models were performed, adjusting for age, sessions, and hemispheres. Inter-agreement of the ALPS index was estimated using Intraclass Correlation Coefficient ICC, considering a two-way model, absolute agreement between rates, and single measures. The ICC provides a quantitative indication of how much of the observed variability is due to actual differences rather than measurement error or rater inconsistency [33]. ICC ranges from 0 to 1, with higher values indicating stronger agreement. It is calculated as the proportion of total variance that is attributable to variability between subjects, relative to the total observed variance (which includes between-subject and within-subject/rater error variance).
In patients with glioma, simple linear regression models have been carried out, adjusted for age, considering the ALPS index as the dependent variable, age as a covariate, and tumor location as a factor (ipsilateral versus contralateral). The grade of the tumor (low versus high-grade) and the sex of the participants have also been considered. A multiple linear regression model has been used to account for all the aforementioned factors.
3. RESULTS
Of the 52 participants in the glioma patient group, 3 were excluded due to bilateral tumor, 3 due to poor signal-to-noise ratio that prevented the use of the images, 1 due to severely altered anatomy from the tumor, preventing the positioning of the ROIs in neither hemisphere, and 1 was excluded because the tumor was not radiologically evident. Therefore, 44 patients with glioma were included in the final analysis. Of all patients, 22 (50%) were women, and the mean age was 40.0 ± 13.9 years. Regarding the grade of gliomas, 24 (55%) were grade II (low grade), 9 (20%) were grade III, and 3 (7%) were grade IV. The grade of the remaining 8 (18%) tumors remained undetermined. Regarding the location of the gliomas, 28 (64%) were in the left hemisphere. Three of the ten healthy subjects scanned in the facilities were women, with a mean age of 25.0 ± 4.0 years for the entire group. Of the 16 healthy subjects from the open dataset, 7 (44%) were women. The mean age of the subjects for the first session was 49.8 ± 18.4 years, and 53.1 ± 17.5 years for the second session.
Table 1 summarizes the ALPS index values in the left and right hemispheres in the two public healthy subjects’ dataset sessions. Data were found to be normally distributed, with an average coefficient of variation of 12.3 ± 2.3% in each session/hemisphere. No significant difference was found between sessions either in the left hemisphere (p=0.636) or right hemisphere (p=0.153). Therefore, data from the two sessions will be considered in the following analysis. The average ALPS index in the left hemisphere was 1.51 ± 0.20, vs. 1.47 ± 0.17 observed in the right hemisphere. No significant difference was found between hemispheres (p=0.150). In other words, the average ALPS value observed in healthy brains was 1.49 ± 0.18. The ALPS index obtained in women was 1.45 ± 0.13, while for men, it was 1.53 ± 0.21. Levene's test for homogeneity indicates that the variances between the two categories are not equal (p < 0.05), so a Welch's t-test was conducted to investigate whether there are significant differences between the sexes. The result of this test was p = 0.09, so there were no significant differences in the ALPS index between women and men. A negative correlation was found between ALPS and age in healthy subjects (p < 0.001, R2 = 0.384), as shown in Fig. (1). A linear model considering the effects of age, hemisphere laterality, sex, and session effects yielded the same conclusion. The only significant difference related to ALPS was observed in age.
The intraclass correlation coefficient between the 4 neuroradiologists was estimated to be 0.34, indicating poor user agreement related to ROI positioning. Comparing indices obtained from each user, average ALPS values ranged from 1.17 (±0.26) to 1.41 (0.31), with coefficients of variation ranging from 17.2% to 28.3%. However, it is worth noting that no significant difference is observed between hemispheres in the evaluations of all the experts.
| - | ALPS | |
|---|---|---|
| Left Hemisphere | Right Hemisphere | |
| Session 1 (N=16) | 1.53 ± 0.18 (1.11 – 1.77) |
1.52 ± 0.19 (1.18 – 1.86) |
| Session 2 (N=16) | 1.50 ± 0.23 (1.02 – 1.80) |
1.43 ± 0.14 (1.19 – 1.68) |
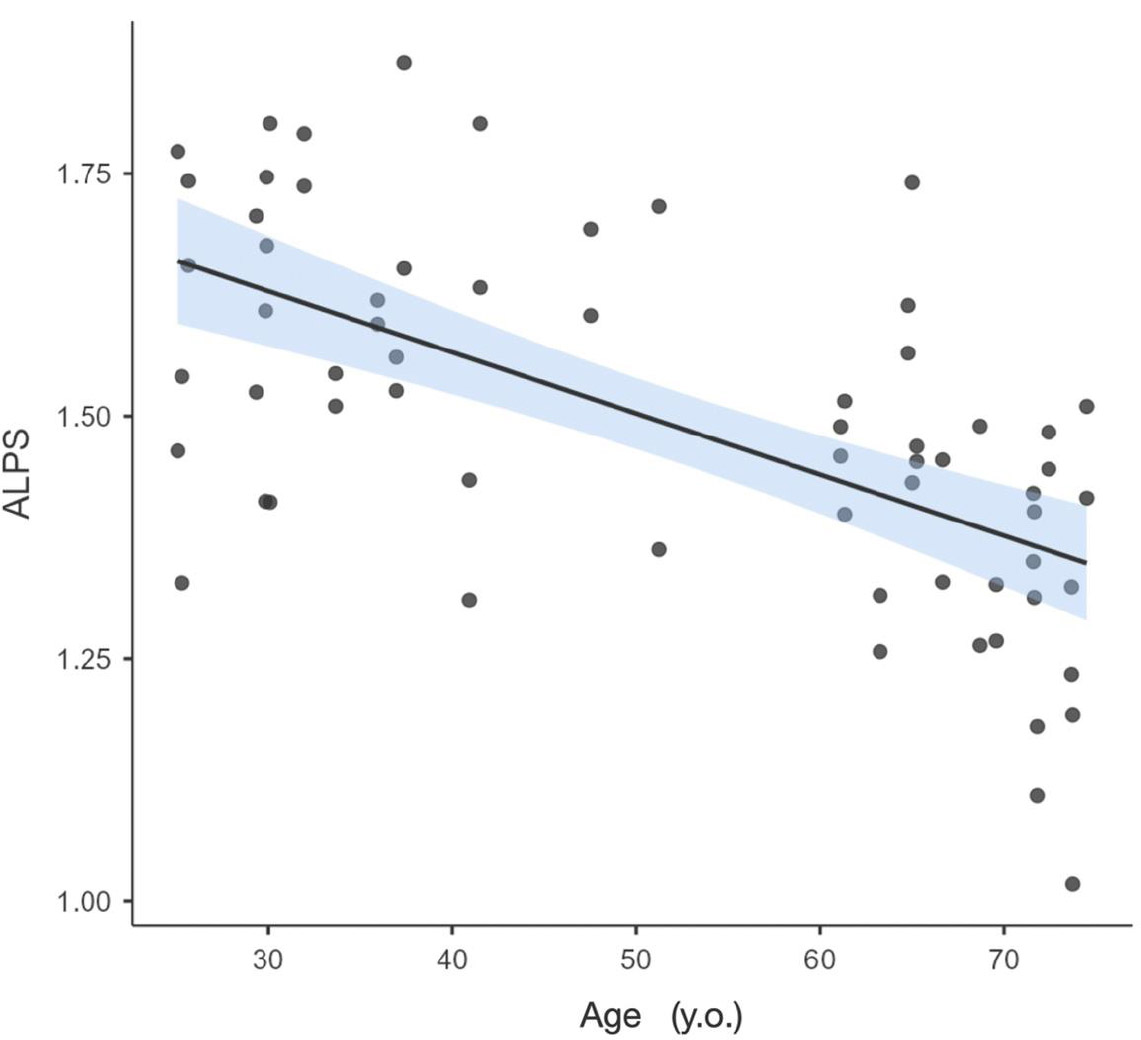
ALPS indices in healthy subjects as a function of age.
In patients with glioma, the average ipsilateral index was 1.46 ± 0.24, while the contralateral value was 1.31 ± 0.22. The ALPS index in healthy volunteers was 1.65 ± 0.20, as presented in Fig. (2). A linear regression of ALPS considering age and hemisphere showed a significant difference between the observed index if measured in the affected hemisphere vs. the non-affected hemisphere (p=0.002), as shown in Fig. (3). Given this difference, the remainder of the study was conducted separately for each hemisphere. No differences related to sex were observed in the ALPS indices in patients with glioma. No significant differences were found between ipsilateral values in glioma patients and healthy volunteers, in contrast to contralateral values, which were found to be significantly different from those of healthy volunteers (p = 0.04, considering all patients, and p = 0.02 in low-grade glioma patients). The results of the age-adjusted linear regression on the ipsilateral hemisphere show a significant difference between low and high-grade gliomas (p = 0.010) while, for the contralateral side, this difference is not significant (p = 0.401). The ALPS value in low-grade glioma in the ipsilateral hemisphere was higher than in high-grade glioma— 1.50 ± 0.27 vs. 1.29 ± 0.26, respectively.
4. DISCUSSION
In this study, the performance of the DTI-ALPS index was evaluated using 1.5T MRI scanners, which corresponds to one of the most common clinical settings. The available hardware inherently influences differences in protocol decisions. For instance, at higher field strengths (e.g., 3T vs. 1.5T), improved SNR can allow for the use of higher b-values and finer spatial resolution, which may reveal subtler tissue contrasts but also increase susceptibility to artifacts or partial volume effects. Conversely, at lower field strengths, compromises in image quality may prompt more conservative parameter choices, potentially affecting the sensitivity and specificity of measurements. Moreover, since DWI (diffusion-weighted imaging) signal behavior is sensitive to both acquisition and post-processing steps, user-driven decisions—such as excluding certain slices, adjusting thresholds, or interpreting low-SNR regions—can compound the variability across datasets. According to the available SNR, decisions are made regarding which b-values to use, with implied diffusion times that reflect different water molecules' displacement ranges. This interplay between hardware capabilities and user-dependent variability underscores a limitation in generalizing results across centers and platforms. This variability could come before the issue of ROI positioning for the estimation of the ALPS index. The range of values observed in this study is consistent with those published at 3.0T. The values observed in healthy subjects are 1.65 ± 0.20, whereas the average values published are 1.50±0.19, with a range of 1.01 to 1.79 in healthy subjects, as presented in Table 2. In other words, the literature indicates that ALPS index values vary across studies, primarily due to differences in acquisition protocols. Conversely, the ALPS index values measured at 1.5T fall within the range of values reported in previous studies conducted at 3.0T.
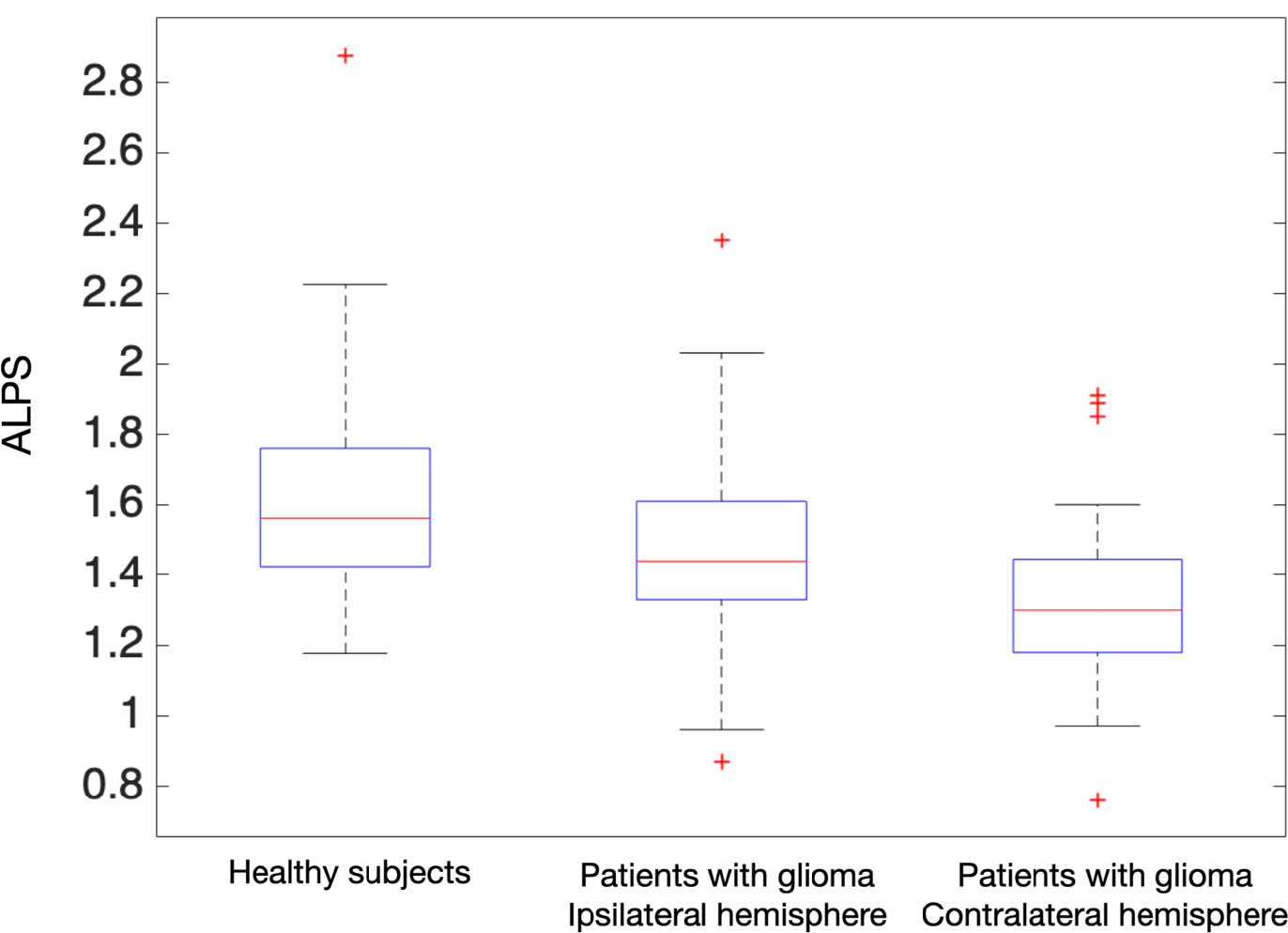
ALPS in healthy subjects and patients with glioma in ipsilateral and contralateral hemispheres.
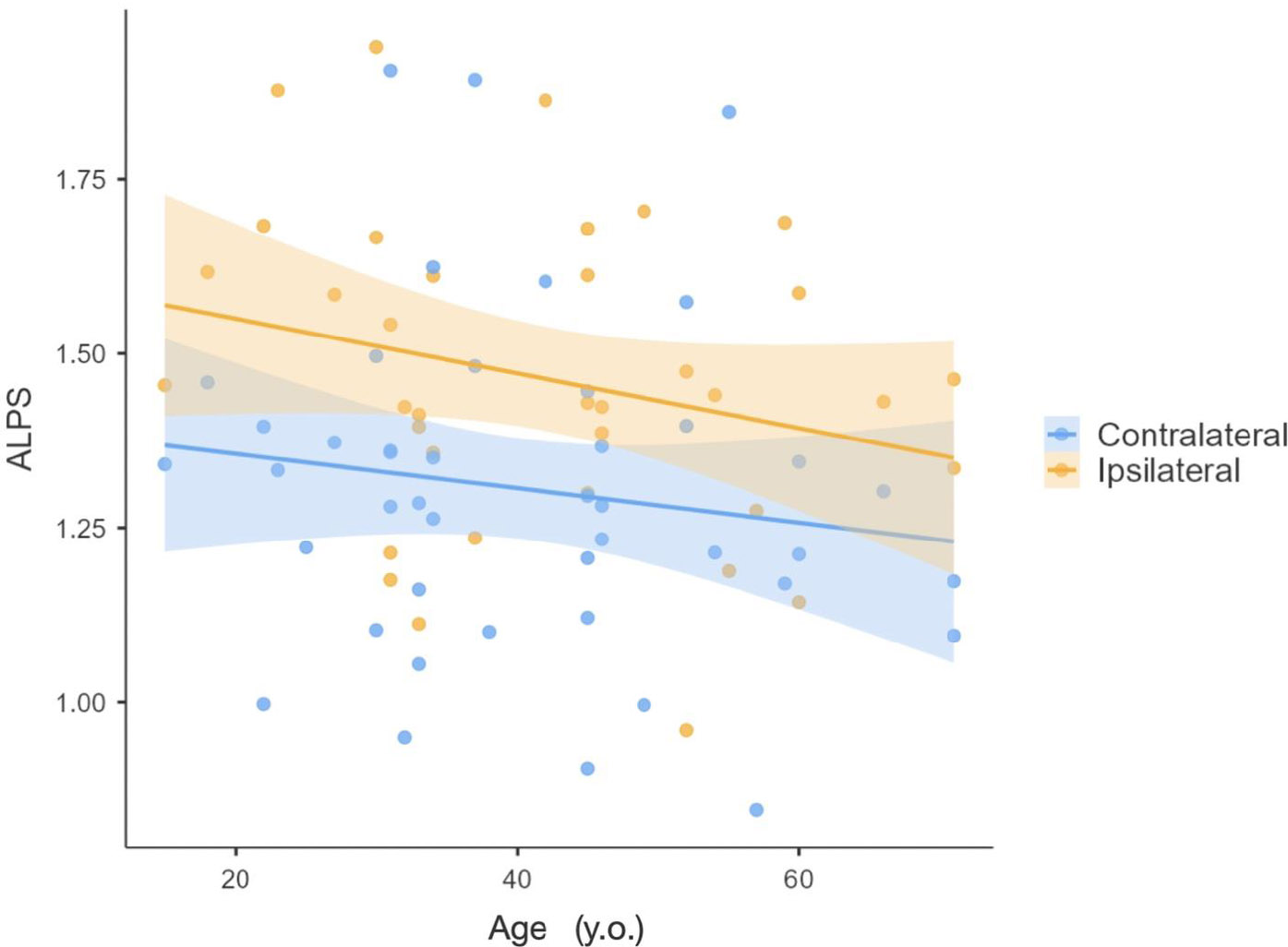
ALPS as a function of age in patients with glioma, in the ipsilateral and the contralateral hemisphere of the tumor.
| Study | Disease | Number of Patients | Number of Healthy Subjects | Hemisphere | ALPS in Patients | ALPS in Healthy Subjects |
|---|---|---|---|---|---|---|
| Steward 20219 | AD MCI |
26 | 10 | Bilateral | 1.45 ± 0.16 1.43 ± 0.16 |
1.48 ± 0.17 |
| Liang 202330 | AD | 38 | 28 | Left | 1.15 ± 0.07 | 1.44 ± 0.07 |
| - | MCI | 18 | - | - | 1.19 ± 0.07 | - |
| - | vascular cognitive impairment | 21 | - | - | 1.21 ± 0.05 | - |
| Yokota 201940 | NPH | 24 | 12 | Left | 1.08 ± 0.03 | 1.18 ± 0.08 |
| Bae 20217 | NPH | 16 | 16 | Left | 1.18 ± ** | 1.54 ± ** |
| Chen 202141 | PD | 88 | 47 | Left | 1.30* | 1.45* |
| McKnight 202124 | PD | 181 | -- | Bilateral | 1.5* | -- |
| Si42 | PD | 168 | 129 | Left | 1.20 ± 0.17 | 1.31 ± 0.17 |
| Lee 202228 | Juvenile Myoclonic Epilepsy | 39 | 38 | ** | 1.54 ± ** | 1.65 ± ** |
| Lee 202243 | Focal Epilepsy | 109 | 88 | ** | 1.67 ± ** | 1.68 ± ** |
| Kim 20245 | Focal Epilepsy | 100 | 79 | Left | 1.55 ± 0.26 | 1.70 ± 0.28 |
| Pu 202344 | Childhood absence epilepsy | 42 | 50 | Left | 1.45 ± 0.36 | 1.66 ± 0.30 |
| Kim 202226 | Cluster headache | 14 | 23 | ** | 1.59 ± 0.37 | 1.79 ± 0.21 |
| Lee 202227 | Migraine | 92 | 80 | ** | 1.65 ± 0.33 | 1.71 ± 0.30 |
| Saito 202323 | Sleep disruption | 317 | 515 | Bilateral | 1.37 ± 0.08 | 1.41 ± 0.08 |
| Hsiao 202325 | Aging (from 13 to 88 y.o.) | — | 433 | ** | — | 1.01 ± 0.06 |
| Yang 202045 | Type 2 Diabetes Mellitus | 20 | 10 | Left | 1.10* | 1.40* |
| Park 20238 | Traumatic Brain Injury | 89 | 34 | Left | 1.32+ | 1.46+ |
| Zhang 202230 | Spontaneous Intracerebral Hemorrhage | 20 | 31 | Bilateral | 1.11 ± 0.23 | 1.67 ± 0.19 |
| Wang 202229 | Cancer Pain Painless Cancer |
67 | 30 | Left | 1.39 ± 0.21 1.52 ± 0.10 |
1.57 ± 0.15 |
| Toh 202111 | Meningioma with peritumoral brain edema Without |
80 | 44 | Bilateral | 1.26 ± 0.17 1.35 ± 0.21 |
1.27 ± 0.10 |
| Taoka 202346 | Patients who underwent whole-brain radiotherapy for brain tumors | 22 | 105 | ** | 1.32 ± 0.16 | 1.44 ± 0.17 |
| Toh 202136 | Glioma | 201 | -- | Ipsilesional | 1.37 ± 0.2 | -- |
| Zhu 202313 | Glioma | 81 | 31 | Ipsilesional | 1.42 ± 0.18 | 1.48 ± 0.17 |
| Average Range |
- | - | - | - | 1.36 ± 0.17 1.08 - 1.67 |
1.50 ± 0.19 1.01 - 1.79 |
This suggests that, despite the expected lower SNR at 1.5T and the longer TE required to achieve the same b-value (i.e., longer diffusion times), the measured underlying biophysical properties remain comparable. Nonetheless, absolute comparisons of ALPS index values between scanners of different field strengths should be made with caution. Standardization of acquisition protocols and calibration across platforms will be essential for establishing robust reference values and improving reproducibility in future clinical applications.
The ICC value observed in this study, at 0.34, is generally considered to reflect poor agreement, suggesting limited consistency among users [33]. Although the ROI placement definition was described in a standard manner for all radiologists, there are inherent operator-dependent variations. Even with standardized guidelines, minor deviations in ROI positioning can lead to significant differences in the ALPS index due to local anisotropy fluctuations. This emphasizes that the DTI-ALPS quantification method remains highly user-dependent, even when all experts agree on the exact descriptive definition for ROI definitions. Therefore, it does not offer high reliability as a quantitative biomarker for individual patients. This result contrasts with published values, where the inter-user ICC between two radiologists was evaluated as ranging from 0.52 to 0.67 on acquisitions at 3.0T [34], and even improved to 0.85-0.94 after image processing for vector reorientation. There are two main differences here— a lower magnetic field was employed, and the ICC was estimated by four radiologists with varying experiences in the field. Another study also emphasized the improvement of reproducibility using image correction processes for tensor vector orientation [35] or directly setting a specific localization on a reference atlas [36]. These observations open the possibility of further improving the inter-user reproducibility values observed in the present study. Yet, the use of normalized space may be questionable in specific pathologies with significant structural differences. Further research is needed to evaluate the use of automatic ROI positioning systems in patients with anatomical disruptions. The use of recent techniques based on deep learning could also be explored with interest [37-39], for their interesting support in the diagnosis of Alzheimer's disease [40] or prediction of epileptic seizure [41]. The present results also highlight the importance of having anatomical reference points to aid in identifying the level where venules run perpendicular to the lateral ventricles, such as with Susceptibility-Weighted Images (SWI) sequences, which were not available on the clinical scanner. Further exploration should confirm that SWI images could improve ROI placement and ICC in DTI-ALPS index estimation.
Another challenge of using this index is that no clear healthy reference value is available, as Table 2 emphasizes. The values reported in the literature vary across studies, ranging from 1.01 to 1.79 in healthy subjects at 3.0T, which is likely due to differences in MRI vendors, specific acquisition parameters (such as chosen b-value, echo time, spatial resolution, ROI definition in terms of size, geometry, and location), and other factors. It has been shown that ALPS indices are larger at shorter TE and that they are affected by the number of diffusion-weighting directions [36]. A local assessment using the same experimental protocol is necessary to compare an altered ALPS value with a healthy reference value. In this work, statistical analysis was conducted comparing patients with healthy subjects, using only data acquired at the institution to avoid mixing data from different acquisition protocols.
On the positive side, no differences are observed between hemispheres in healthy subjects, whereas such differences are apparent in patients with glioma, as demonstrated in this study. The ALPS values are also reproducible between sessions with coefficients of variation close to 10%, which is promising in the sense that it should be sufficient to distinguish an alteration in ALPS in case of pathology. The influence of sex on glymphatic function continues to be an active research subject. Men tend to have a larger diameter of the arteries and a greater dilation of the perivascular space than women. Whereas, women tend to have a higher blood velocity in the common carotid arteries. The combination of these factors could nullify the effect of each one and produce comparable glymphatic drainage between sexes [42]. On the other hand, there is a significant negative correlation between ALPS and age, which indicates a deteriorated glymphatic system with age. It appears that glymphatic function could decline with aging due to several factors. Studies in aged animals have shown that the reduction of aquaporin-4 channels at the ends of astrocytic cells decreases CSF production and CSF pressure, and decreased arterial pulsatility may lead to compromised glymphatic function [42]. Age-related changes in the glymphatic system represent a key factor that may influence ALPS index values, and failure to account for these variations can act as a potential confounder, particularly in clinical applications. As such, age must be carefully considered when interpreting ALPS values, especially when comparing individuals of different age groups or using absolute values in diagnostic or prognostic contexts. Moreover, it is essential to note that, to date, there is no universally accepted normative range of ALPS values in healthy individuals, partly because ALPS index values appear to be sensitive to differences in acquisition protocols. These methodological variations further complicate cross-subject or cross-study comparisons without appropriate normalization or control strategies. To address this issue, intra-individual contralateral comparisons are emphasized, particularly when assessing hemispheric differences. This strategy allows us to partially mitigate the confounding effect of age, as both hemispheres within a single individual are subject to the same age-related background changes. This within-subject control helps isolate localized alterations in glymphatic flow that may be pathologically relevant.
In the case of the observations in patients, it has been proposed that tumor growth alters the balance between periarterial CSF inflow and perivenous interstitial fluid outflow and results in interstitial fluid accumulation [11, 12, 43]. An increased glymphatic function may facilitate the removal of interstitial fluid, reducing or even preventing edema. Conversely, insufficient glymphatic function for removing interstitial fluid may contribute to edema formation. In the present study, the effects of glioma on ALPS were indeed observed, as the index values differed between the affected and non-affected hemispheres, as expected, emphasizing that this index could function as a biomarker of an impaired glymphatic system in a conventional clinical context, evaluated with 1.5T MRI scanners. These observed differences emphasize that the control of the glymphatic system is differentiated by hemisphere, as suggested by Zhang et al. in a study of patients with spontaneous intracerebral hemorrhage [30]. The values observed here indicate a decreased glymphatic function in the contralateral hemisphere compared to healthy volunteers, suggesting an altered clearance system in the organ affected systemically, not only locally in the ipsilateral hemisphere.
Preclinical studies show alterations in the contralateral hemisphere when glioma-infiltrated cortical regions are present, for example, the bilateral synchrony of spontaneous neuronal activity gradually decreases, while neurovascular coupling becomes progressively disrupted compared to the uninvolved cortex [44]. Additionally, in gliomas, there are affected brain regions without evident signal alterations in MRI structural sequences, which may still be infiltrated. The diffusion along perivascular spaces in the contralateral side is abnormally reduced in the patients with glioma, in contrast to the work published this year by Villacis et al. [45]. This could reflect tumor dissemination, global metabolic dysfunction, and neuroinflammation associated with insufficiency in clearing waste products.
Interestingly, many published studies focus only on one hemisphere, as reported in Table 2. However, it would be valuable to further evaluate this potential differentiated control, particularly with different pathological conditions, whether focused or not, or to leverage the two possible measurements to obtain a more robust estimation of the ALPS index itself.
The findings regarding the relationship between the ALPS index and the grade of the tumor are intriguing, as previous studies have not reported differences in the DTI-ALPS index based on tumor-type [45]. In low-grade gliomas, only the contralateral ALPS values are decreased compared to the healthy values observed in this study, whereas in high-grade gliomas, both hemispheres exhibit lower values. It can be assumed that the glymphatic system initially decreased its activity in the contralateral hemisphere to compensate for the imbalance caused by tumoral infiltration or other fluid alterations that may be diffuse or bilateral and are not visible to conventional MR sequences. In more aggressive tumors, the glymphatic system may be unable to sustain the activity required to restore interstitial fluid flow, affecting both hemispheres.
5. LIMITATIONS
A limitation of the present study is that the sample size of healthy subjects was relatively small and limited to younger individuals, due to the difficulty in recruiting volunteers without any underlying pathological conditions and limited access to scanner time for scanning asymptomatic participants. As ALPS varies with age, this may also be a confounding factor for comparing the different published values if the age characteristics of each patient population are different. On the other hand, too few high-grade glioma data were included in the present analysis, and further studies are needed to increase the number of bilateral observations in both patients and healthy subjects, in relation to different acquisition protocols. It is worth noting that these findings are coherent with the measures by other groups where the ALPS index was significantly higher in lower-grade gliomas than in higher-grade gliomas [13, 43].
CONCLUSION
In this study, the usability of the Diffusion Tensor Image Analysis Along the Perivascular Space (DTI-ALPS) index was evaluated as a biomarker for the glymphatic system using 1.5T MRI. The high variability observed between users indicates that it is not yet suitable as a standalone quantitative biomarker for individual clinical assessments. The study also highlighted a significant difference in ALPS index values between the ipsilateral and contralateral hemispheres in glioma patients, with the ipsilateral side showing values closer to those of healthy subjects. This suggests that the ALPS index could be sensitive to pathological changes, specifically glioma-related alterations in the glymphatic system. From a clinical perspective, these findings emphasized that the DTI-ALPS index cannot be considered a reliable standalone biomarker due to its protocol dependence and age-related limitations. Considering microangiopathic damage in the context of physiological aging or the high prevalence of risk factors for microvascular damage, it would be ideal to have standardized data from population-based studies conducted with comparable acquisition and post-processing protocols. Given the apparent limitations of this approach, an alternative could be to compare patients of similar age, adjusting for vascular risk factors and the presence of microangiopathic damage or signs of tumor infiltration visible on T2-weighted FLAIR sequences. One of the interesting points that presents direct implications from a clinical perspective is using the patient's contralateral hemisphere value to contrast the evaluation for each patient, particularly in cases of focal pathologies such as the presence of a tumor.
However, considering the limitations and criticisms of the model [46], it is believed that the ALPS index is an interesting non-invasive method for evaluating the glymphatic system, even at 1.5T MRI, particularly due to its ease of implementation. Several future research directions are emerging. Firstly, there is a clear need for the standardization of both acquisition and postprocessing protocols for DTI-ALPS, as current methodological variability—across centers and magnetic field strengths—limits the generalizability of results. Developing harmonized pipelines would enable more reliable comparisons and facilitate the establishment of normative reference values. In parallel, building population-based normative databases stratified by age, sex, and vascular risk factors could provide essential context for interpreting ALPS values, especially in clinical settings. Another promising direction involves systematically investigating hemispheric asymmetry in ALPS values, particularly in focal pathologies such as gliomas, where contralateral referencing may enhance diagnostic specificity and accuracy. Future studies should also focus on developing automated or semi-automated ROI placement algorithms in the context of pathological anatomy to address the high inter-user variability observed in this study, thereby improving reproducibility and facilitating broader clinical adoption. Finally, cross-field strength validation studies comparing ALPS measurements obtained at 1.5T and 3.0T using harmonized protocols could clarify how field strength influences ALPS reliability, helping define the role of lower-field MRI systems in glymphatic imaging. By addressing these challenges, the DTI-ALPS index has the potential to become a valuable non-invasive tool for assessing glymphatic system function and its role in various neuropathological conditions.
AUTHORS’ CONTRIBUTIONS
The authors confirm their contribution to the paper as follows: F.T., R.R., P.C., S.C.: Study conception and design; J.V., S.R., M.G., C.B.: Data collection; I.M., B.O., C.S.: data Analysis or Interpretation. All authors reviewed the results and approved the final version of the manuscript.
LIST OF ABBREVIATIONS
| AD | = Alzheimer’s Disease |
| ALPS | = Analysis along the Perivascular Space |
| CSF | = Cerebrospinal Fluid |
| DTI | = Diffusion Tensor Imaging |
| FA | = Fractional Anisotropy |
| FLAIR | = Fluid-Attenuated Inversion Recovery |
| FSPGR | = Fast Spoiled Gradient Echo |
| HGG | = High-Grade Glioma |
| ICC | = Intraclass Correlation Coefficient |
| LGG | = Low-Grade Glioma |
| MCI | = Mild Cognitive Impairment |
| MRI | = Magnetic Resonance Imaging |
| NPH | = Normal Pressure Hydrocephalus |
| PD | = Parkinson’s Disease |
| ROI | = Region of Interest |
| SWI | = Susceptibility Weighted Images |
| TE | = Echo Time |
| TR | = Repetition Time |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study received approval from our local ethics committee (Servicio de Salud Valparaíso San Antonio, Chile CEC-SSVSA #72/2017).
HUMANS AND ANIMALS RIGHTS
All human research procedures followed were in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013.
AVAILABILITY OF DATA AND MATERIALS
The healthy subject data supporting the findings of the article in the test-retest condition is available in the OpenNeuro repository at https://openneuro.org/, reference number ds001378.
FUNDING
This work was funded by ANID - Millennium Science Initiative Program - ICN2021_004 and by Fondecyt grant#1231268 (SC).
ACKNOWLEDGEMENTS
Declared none.


