All published articles of this journal are available on ScienceDirect.
Caudate Nucleus and Insular Activation During a Pain Suppression Paradigm Comparing Thermal and Electrical Stimulation
Abstract
Pain modulation is an integral function of the nervous system. It is needed to adapt to chronic stimuli. To gain insights into pain suppression mechanisms, two studies concerning the suppression of the feeling of pain with different stimulation modalities (heat vs. electrical stimuli) but using the same stimulation paradigms were compared: 15 subjects each had been stimulated on both hands under the instruction to suppress the feeling of pain.
Anterior insula and DLPFC activation was seen in both single modality studies and seems to be a common feature of pain suppression, as it is absent in the interaction analyses presented here.
During the task to suppress the feeling of pain, there were no consistent activations stronger under thermostimulation. But during electrostimulation, there was significantly stronger activation than during thermal stimulation in the caudate nucleus bilaterally and in the contralateral posterior insula. This may be attributed to the higher sensory-discriminative content and more demand on subjective rating and suppression of the painful electrical stimulus, compared to thermostimulation. The caudate nucleus seems to play an important role not only in the motor system but also in the modulation of the pain experience.
INTRODUCTION
While the perception of acute pain is essential to ensure the integrity of the organism [1], most chronic pain is useless [2] and may cause disabling illness. The modulation of painful stimuli is accomplished by peripheral as well as spinal and cerebral up and down regulation [3] of the nociceptive inputs. A diminished capacity to suppress pain may be hypothesized to predispose to chronic pain syndromes.
The processes underlying pain suppression are therefore interesting in this context.
There are many ways to simulate clinical pain in experimental conditions:
Noxious chemical stimulation [4, 5] or muscle ischemia [6] have been used. Noxious heat stimulation can be administered by a thermode [7-12] or by a laser [1, 13, 14]. Electrical stimulation [14-19] or mechanical stimulation [20, 21] have also been used.
The conduction of the stimuli generally has been differentiated into two neural pathways: Medial and lateral pathways [22, 23] are distinguished.
Receptors that are sensitive to pain and temperature belong to the medial pain system. They transduce via Aδ and C-fibres [24] to the contralateral spinothalamic tract and further on via medial thalamus to the anterior cingulate cortex (ACC), periaqueductal grey (PAG) and insula to the prefrontal cortex [25]. The latter are responsible for affective and emotional aspects [26] of the pain experience.
The lateral pain system transduces input from low threshold mechanoreceptors (touch and sense such as vibration and kinaesthesia) [24] by means of Aα and Aβ fibres to the medial lemniscus and on to thalamus and somatosensory cortex, dealing with sensory-discriminative aspects [22].
Our special interest was to explore the cerebral regions involved in pain suppression. Own studies with electrical [18] and thermal [7] stimulation show differing results: During the task to suppress the feeling of electrically induced pain, subjects showed cerebral activation in prefrontal cortex and caudate head and thalamus. In the same task during thermal stimulation, however, caudate, insular and prefrontal cortex activation was detected. However, no formal statistical comparison had been done yet.
There already have been pain studies comparing different stimulation modalities [6]. The only fMRI study comparing thermal and electrical stimulation, however, found no relevant cerebral activation during thermal stimulation [12].
Since functioning pain suppression is supposed to be a prerequisite for wellbeing despite repeated noxious inputs, the underlying mechanisms have to be understood in healthy subjects. Thus the results of studies on thermal and electrical stimulation during the task to suppress the feeling of pain are now compared, both on a functional and on a formal, i.e. statistical, level. We hypothesized that the different properties of the stimulation method should manifest in different cortical activation.
METHODS
Single Modality Studies
Since the results of the single modality studies have already been reported in detail [7, 18], only a short summary of the procedures is given.
Subjects
After approval of the local ethics committee and in accordance to the Declaration of Helsinki, 15 healthy, right handed volunteers had been recruited for each arm of the study.
Data Acquisition
FMRI data were acquired on a 1.5T MR scanner (Magnetom Symphony, Siemens, Germany).
Blood Oxygen Level Dependent (BOLD)-contrast was measured with an EPI sequence with 28 axial slices of 5mm thickness, 10% gap, and field of view 230mm. TR was 2600ms, TE 60 ms, Flip angle 90°. Resolution was 64 x 64. We used Cartesian read-out and a band-width of 2442 Hz/ Px.
Anatomical data were acquired using a sagitally oriented T1 weighted MP RAGE 3D sequence (magnetization prepared rapid acquisition of gradient echo [27] equivalent to a fast SPGR-sequence) with isotropic 1mm³ voxels and a T2 turbo spin echo sequence (TR 2530ms, TE 99ms, FOV 230mm, matrix 256) with the same slice orientation as the BOLD Sequence.
Experimental Protocols
The subjects were stimulated on their index finger tips on both sides subsequently; the order was assigned randomly.
Because of high interindividual differences of skin thickness and susceptibility to pain, individual thresholds established directly preceding the fMRI-experiment were used. The stimulus intensities were tested in a ramp style pattern with slowly rising stimulation levels as described in detail [7, 18] with the experimenter and the subject facing each other outside the scanner room. The level was readjusted again in position in the scanner to take into account the change of pain threshold during distraction [28].
Rest: no stimulation / indifferent temperature.
Pain: the strongest painful stimulation the subject is able to endure for up to 52s.
For fMRI the protocol was presented on a PC, running ERTS (BeriSoft AG, Frankfurt, Germany) synchronized with the MR-scanner.
Electrical stimulation was administered to the index finger via 2 MRI-compatible adhesive ECG skin electrodes, placed 5 cm apart, by an electroneurograph (Myograph DA1 Tönnies, Freiburg, Germany) positioned outside the scanner room. The active electrode was placed at the volar tip of the index finger.
The pulse duration was set to 0.2 ms, 10 pulses per second.
Thermal stimulation was presented by a MRI-compatible Peltier thermode with a stimulation area of 30x30mm, on which the volar tip of the index finger was placed. The generator (Thermal Sensory Analyser II, Medoc Advanced Medical Systems, Rimat Yishai, Israel) was positioned outside the scanner room. The stimulation was applied with rising temperatures, until the desired maximal tolerable pain was reached.
Stimulation Paradigms
The experiment was set to investigate cortical correlates of suppression of tonic painful stimuli.
The stimulation levels were chosen after another experiment with different stimulation levels. The maximally tolerated pain, however was the level for which both thermal and electrical stimulation aimed in this study. The tonic painful stimulation used stimulus duration of 52 s of maximum intensity with the task to suppress the feeling of pain. It started with a rest phase (26 s) before each stimulus phase. The whole set consisted of 6 repetitions of rest and stimulation. (see Fig. 1).
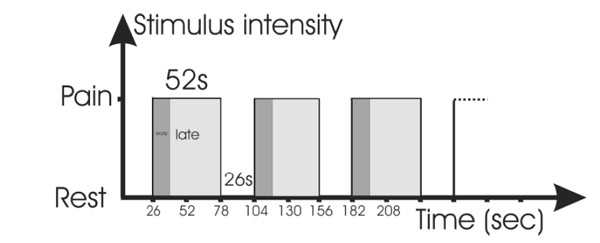
Stimulation paradigm during the trials: Alternating phases of rest and painful stimulation. The tonic stimulation was divided into an early and late phase for purposes of analysis; the stimulation, however, was the same.
For the purpose of analysis, the tonic-painful stimulation paradigm was divided into the first 13s and the following 39s (tonic pain early, abbreviated tpe and tonic pain late, tpl). The duration of the early phase was deducted from experience in pre-trials, where the time to achieve suppression of pain was between 10 to 15 seconds which is similar to the early phase reported by others [29].
Suppression of Pain
The subjects had to suppress the feeling of pain every time it arose. They were free to choose the technique for suppression. Some examples were given: Mental imagery (“Think of your last holiday”), depersonalisation techniques (“imagine extending your finger and shoving the pain away from you”) or distracting by other means.
Rating the Pain Experience
Although there have been recommendations made for simultaneous rating [9], own experience [30] showed the possibility of import of motor task related activation into an otherwise sensory paradigm. Therefore we decided to rate at the end of the session after the experiment. Subjects were familiar with a four level rating scale from a previous experiment using graded stimuli: The levels were modelled after established “anchors” [31] and were explained as follows:
Level 1: No stimulation (This corresponds to the rest condition),
Level 2: A slight sensation, securely above the perception threshold,
Level 3: A strong sensation, but not painful,
Level 4: Pain.
The levels of subjective experience (pain level) after suppression of pain under constant stimulation were reported verbally immediately after termination of the experiment.
Data Analysis
Differences regarding psychophysical or demographic parameters were computed using the t-test and Mann-Whitney test with p< 0.05 as a threshold for significance.
For the fMRI analysis of the trials and the group comparison, SPM 5 (Wellcome Department of Imaging Neuroscience, London, UK) [32, 33] was used. We used the scanner-inherent motion correction together with the motion correction of SPM. Afterwards the scans were normalized to the MNI-Template [34] and smoothed with an isotropic Gaussian kernel of 8mm full-width at half maximum. Standard high- and low-pass filtering of SPM was used. For high-pass filtering: Session cut-off period was set to 156s. The low-pass filter was set to the option hrf (hemodynamic response function).
A group analysis using the random effects model was conducted to avoid problems with group comparisons [35]. The cluster size had to exceed 10 voxels, a voxel threshold of p<0.001 uncorrected was applied to the resulting datasets; only activation appearing during both right and left hand stimulation (for lateralized functions the contralateral activation also counted) on the maps exceeding this threshold were taken to be significant.
Areas of activation were identified with the help of the Talairach Daemon [36].
The contrasts are defined as follows:
Primary contrasts, they exist for right and left side stimulation:
Tonic pain early versus rest: Tpe-r (this refers to the first 13 seconds).
Tonic pain late versus rest: Tpl-r (including the 14th to 52nd second).
Secondary contrasts, they refer to the comparison of thermal and electrical stimulation:
Areas activated more in thermal than electrical stimulation: T-E.
Areas activated more in electrical than thermal stimulation: E-T.
The presented interaction analyses uses a primary and a secondary contrast in conjunction: it takes data from a primary contrast and explores which areas are more active during thermostimulation or electrostimulation.
RESULTS
Biometrical and Psychophysical Data
The group undergoing electrical stimulation comprised 10 males and 5 females, aged 25-64 years (mean 35.5 y). The thermally stimulated group consisted of 8 males and 7 females, aged 19 to 47 years (mean 32.8 y). The age difference was not significant (t-Test, p=0.45).
The perception during the suppression of the tonic painful stimulation was rated with 2 as a median both during electrical and thermal stimulation (Mann-Whitney test, not significant, p=0.77).
fMRI Results
Areas activating more in electrostimulation than during thermostimulation:
Comparing the two stimulation modalities, several areas had significantly stronger activations during electrical stimulation than during thermostimulation. In the early phase (contrast Tpe-r), multiple foci of activation bilaterally in the whole caudate nucleus and posterior insula were detected (see Table 1a) and Fig. 2).

Cortical activation in the early phase of the tonic stimulation that was stronger during electrical than thermal stimulation (contrast e-t (tpe-r)).
Diagonal arrows point to activation in the caudate, while the horizontal arrows point to the contralateral posterior insula. The figures are presented in neurological convention, the subjects´ right side being on the right side of the figure. The labels “right” and “left” refer to the stimulated side.
In the late phase (contrast Tpl-r), only contralateral posterior insular activation was consistently activated (Fig. 3).
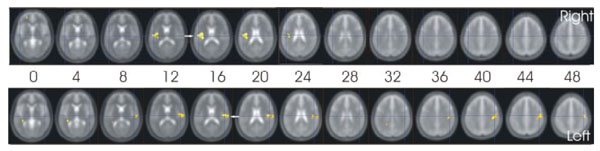
Cortical activation in the late phase of the tonic stimulation that was stronger during electrical than thermal stimulation (contrast e-t (tpl-r)).
The arrows point to the activation in the contralateral posterior insula.
The figures are presented in neurological convention, the subjects´ right side being on the right side of the figure. The labels “right” and “left” refer to the stimulated side.
Areas activating stronger during thermostimulation than during electrostimulation:
Here, in the early phase, several areas showed activation (see Table 1b)). However, since these areas were activated inconsistently (only during left or right hand stimulation), they are regarded as not relevant.
Single modality data (electrostimulation only or thermostimulation only) are available as supplementary material in Table 2.
DISCUSSION
The subjects of both studies had succeeded similarly to suppress the feeling of pain during tonic stimulation with different modalities. However, statistical comparison of the brain areas activated during the suppression task showed significant differences: During electrical stimulation, caudate nucleus and posterior insula showed stronger activation than during thermal stimulation.
Posterior Insula
Conscious pain processing and subjective evaluation of heat pain have been shown to correlate with activation in the anterior insula [37, 38]. This activation has been present in our single modality studies [7, 18], but seems to be common to both, so that it is absent in the exploration of the differences undertaken here.
The objective intensity of a heat pain stimulus correlated with activation in the posterior insula [37]. Other studies had shown posterior insular activation, when attention was diverted from the painful stimulus [39] or during comparison of controlled and uncontrolled pain [40].
The current data show that during the task to suppress the feeling of pain, posterior insular activation is stronger in the electrostimulation experiment. This may be interpreted as a direct effect of the different stimulus properties: Electrostimulation is known to excite predominantly low threshold mechanoreceptors as well as Aα and Aβ fibres, resulting in input to the SI and SII via lateral pain system [22, 41]. Contrarily, thermostimulation results in selective activation of Aδ and C fibres, resulting in input to the ACC and anterior insula via medial pain system [26].
The stated physiology in turn results in more sensory-discriminative processing via lateral pathway during electrostimulation and may be responsible for the predominant activation of posterior insula (SII) during electrostimulation. Posterior insula activation has been linked to sensory discriminative aspects of pain perception [42, 43] and predominantly sensory and motor connections that include SI and SII and motor and premotor areas [44].
Similarly, in a comparison between impact and thermal pain [43], more pronounced posterior insular activation during impact stimulation has been shown. It has been speculated to originate from stronger mechanoafferent input during the ballistical stimulation.
Laterality
Many studies show right insular activation during painful stimulation [40, 45], while in our study, the activation seems to be localized contralaterally, as has been reported for thermostimulation [39, 46]. However, this may be due to the subtraction process: We show that contralateral insula activates in E-T, while the other studies concentrated on single modality perception. So, perhaps the distinguishing factor of electrical stimulation (more sensory-discriminative components) results in contralateral insular activation that has shown to be a feature of the subjective rating process [38].
Caudate Nucleus
In recent studies, activation of the caudate nucleus has been shown during control and suppression of stimuli [47, 48], especially of pain [7, 18]. Activation of the caudate nucleus contributes to eliciting or suppression of specific patterns of motor behaviour in response [48], is activated during evaluation of the spatial locations of noxious stimuli [49] or during expectancy of pain [50]. Furthermore, caudate activation has been shown during mechanical [51], but not thermal stimulation [52]. Also, lower activation has been detected in the caudate nuclei of patients with fibromyalgia [53] or chronic fatigue [54]. To summarize, the caudate nucleus seems to play an important role in both the sensory processing and suppression of pain. A lack of caudate activation is associated with chronic pain or fatigue.
In the present study, caudate activation is a distinguishing feature in the early phase of the tonic painful electrostimulation during the task to suppress the feeling of pain. Together with the recent reports of the literature cited above, the caudate activation therefore is interpreted as a feature of the task to suppress the feeling of pain. This may be due to the effort [7] needed for suppression.
Another explanation could be the higher sensory-discriminative content of electrically induced pain. Before it is suppressed, therefore, more sensory processing takes place, so that this stronger caudate activation could be interpreted as a correlate of this sensory activity.
The activation shown in Fig. (2) seems to extend into the ventricle. This, however may be due to the process of smoothing. The activation has been labelled with the Talairach daemon.
GENERAL CONSIDERATIONS
Success of Suppression
In both the electrically and thermally stimulated experiments, subjects felt able to suppress the feeling of pain. They reported similar levels of pain perception during suppression, regardless of the stimulation modality. The differing cortical activation therefore must be attributed not to the pain level, but to other aspects of the pain perception or to the suppression process itself. However, since the reporting of the suppression effect was done after the cessation of the experiment, the results could be confounded by pain memory differing from the real sensation.
Missing Homogeneity of the Study Population
Since the studies were not performed on the same subjects, there is the danger of inter subject differences taking precedence over the studied variables. However, since we undertook only interaction analyses (we compared the differences, e.g. Tpe-r and not the raw values), the influence of the subject is minimized.
Possible Anticipatory Activation
Since the time course of the stimulation paradigm was fixed in the experiments, subjects could anticipate the next stimulation course. However, the task was to suppress the feeling of pain when it arose, and not to eliminate it altogether. Own subjective experience agrees with that: The painful stimulation every time came like jolt; to accomplish suppression took several seconds. Caudate activation has been shown during expectancy of pain [50]. However, since the expectancy is thought to be the same in both experiments regardless of stimulation modality, this (common) effect should be eliminated in the interaction analysis focussing on the differences between the modalities presented here.
Thresholds
The experiments were performed on both sides with a threshold for the single evaluation of 0.001 uncorrected.
Only activation present during stimulation of both sides was accepted: Coordinates on the y- and z-axis had to match. Identical x-axis coordinates during both sides of stimulation mean a lateralized function, whereas opposite x-axis coordinates point to activation of the contralateral (or ipsilateral) side.
By asking for activation to occur during both right and left side stimulation, a rather conservative exploration was performed.
Differentiation of the Stimulation Phases
For the purpose of analysis, the tonic stimulation phase has been divided, as has been reported by others [55], who attributed late phase changes to opiate mediated effects. The duration of the early phase was deducted from experience in pre-trials, where the time to achieve suppression of pain was between 10 to 15 seconds which is similar to the early phase reported [29].
Missing Activation of Dorsolateral Prefrontal Cortex (DLPFC)
Frontal lobe activity generally has been related to cognitive and attentional processing of painful stimuli [56] or to reappraisal [57].
DLPFC activations have been found in our studies in single modality analysis [7, 18], but they were not found in the presented interaction analysis because activation due to the same task in both studies is subtracted. Seemingly, the effort of suppression therefore is similar during both electrical and thermal stimulation. This lends power to the second argument favouring the sensory content of the stimuli as the cause for caudate activation during electrostimulation.
CONCLUSIONS
Anterior insula and DLPFC activation was seen in both single modality studies and seems to be a common feature of pain suppression, as it is absent in the interaction analyses presented here.
There were no consistent activations stronger during the suppression of pain under thermostimulation. But during electrostimulation, there was activation significantly stronger than during thermal stimulation in the caudate nucleus bilaterally and in the contralateral posterior insula. This may be attributed to the higher sensory-discriminative content and more demand on subjective rating and suppression of the painful electrical stimulus, compared to thermostimulation.
SUPPLEMENTARY MATERIAL
Supplementary material is available on the publishers Web site along with the published article.


