All published articles of this journal are available on ScienceDirect.
Diagnosis of Lymphatic Metastasis in Breast Cancer Using Nanoparticle Technology - Diagnosis, Therapy, Imaging, Treatment
Abstract
Breast cancer is a major cause of patient death rates, frequently leading to life-changing repercussions even after survival is attained. This paper aims to investigate therapeutic alternatives employing nanoparticles to specifically target and treat lymphatic metastasis, which is a highly dangerous characteristic of breast cancer. This work explores the effectiveness and importance of using nanoparticle-based therapeutics to prevent the harmful consequences of breast cancer progression. The paper begins by discussing the progress of lymphatic metastasis and then delves into the use of nanoparticle technology in imaging techniques, diagnostic methods, and therapy tactics. This section provides detailed information on primary targeting treatments, including chemotherapy specifically targeting cancer stem cells, induction of tumour cell death, suppression of Epithelial-Mesenchymal Transition (EMT), manipulation of the Tumour Microenvironment (TME), and improvement of the immune response. In addition, the research explores the use of nanoparticle technology in treatment plans, specifically focusing on its super magnetic capabilities and the application of gold nanoparticles, nanodiamonds, and other related qualities. Nanoparticle technology presents an optimistic strategy to address lymphatic metastasis in breast cancer. Nanoparticles can be used to deliver drugs or therapeutic substances directly to cancerous tumours, specifically targeting cancer cells to either destroy them or slow their growth. This strategy provides a solution for the administration of pharmaceuticals or substances that may provide challenges when delivered using conventional methods. Furthermore, nanoparticles facilitate the visualisation of tumours, aiding healthcare professionals in evaluating the severity of malignancy and formulating suitable treatment strategies. A comprehensive discussion has been conducted on several nanoparticles employed for inhibiting the dissemination of cancer cells from the primary organ to secondary organs. After successfully overcoming breast cancer, patients remain susceptible to post-surgical metastases in vital organs such as the lungs, brain, and bones. The advancements achieved through nanoparticle technology are highly significant. The discussion has focused on experimental evidence offered by researchers who mostly conducted studies on mice to support their findings.
1. INTRODUCTION
Breast cancer is a prime example of how treatment methods have changed as a result of an increased understanding of the biological disease condition. Without compromising oncological care, the ultimate goal of any treatment process is to protect the local anatomy of the body and improve Quality of Life (QOL) [1]. Cancer is the most dangerous non-communicable disease known to man. The uncontrollable growth of normal body cells to form abnormal clumps of tissues called tumors is cancer. The most feared concept of cancer that goes unnoticed, leading to the various harmful stages of the disease itself, is metastasis [2]. Cancer cells spread throughout the body through a variety of mechanisms, including invasion and migration from the main tumor, intravasation and survival in the bloodstream, extravasation into distant tissues, and the establishment of metastatic foci in the seeded organs [3]. In the United States, breast cancer is the most common type of cancer and the fourth-leading cause of cancer-related mortality [4]. Metastasis can be related to “soil” formation and “seed” dissemination. The “seed” cells are transported from the primary tumor (the body part that shows cancer initially in stage one). The premetastatic niche, the “soil,” in a distant organ, prepares for the establishment of the disseminated “seed” cells. Breast cancer is perfect in the sense of representing this phenomenon through lymphatic metastasis [5]. According to studies, tumors under hypoxic conditions are more likely to produce these “seed” cells. A hypoxic microenvironment is created by the imbalance of less oxygen available to the cells and the overconsumption of oxygen by the cells during cell multiplication as the malignant tumor takes form. The oxygen deficit pertains to the cancer-associated fibroblasts. These cells are transported through the victim’s bloodstream to other parts of the body. It then develops an environment for the “soil” to form in this new cancer site. It becomes a secondary “soil” region by the secretion of PMN-fostering factors to the metastatic sites. In a simple form, demeaning the hypoxic conditions in the primary tumor will reduce the risk of metastasis. Thus, in one way, the basic idea is to prevent the seed soil hypothesis from taking over the cancer-infected patient’s body.
Nanoparticle technology presents a promising approach to the advancement of therapeutic strategies for many disorders, such as cancer. Nanoparticles, with sizes typically ranging from 1 to 100 nanometers, possess distinct characteristics that render them appropriate for precise medication administration and imaging purposes. Important elements of nanoparticle technology for treatment programmes include targeted drug delivery, enhanced pharmacokinetics, controlled release, multimodal imaging, and personalized medicine. Nanoparticles can be designed to include medicinal substances like chemotherapeutic medicines, antibodies, or nucleic acids, enabling precise delivery of these agents to specific targets. These nanoparticles can be engineered to selectively target cancer cells or tumor tissues while minimizing harm to healthy cells, reducing side effects, and improving the effectiveness of treatment.
Nanoparticles possess increased pharmacokinetics due to their small size and surface features, allowing them to avoid elimination processes in the body and specifically accumulate in tumours by using the Enhanced Permeability and Retention (EPR) effect. This mechanism enables extended circulation durations and enhanced medication concentrations at the tumour location. Nanoparticles can be engineered to release their contents in a controlled manner when exposed to certain stimuli, such as changes in pH, temperature, or enzyme activity. The controlled release method enables exact control over the rate at which the medicine is released, ensuring that the therapeutic effects are optimized while minimizing any harmful effects on the body as a whole. Nanoparticles can function as contrast agents for multiple imaging techniques, such as Magnetic Resonance Imaging (MRI), Computed Tomography (CT), Positron Emission Tomography (PET), and optical imaging. Through the process of conjugating imaging probes to nanoparticles, scientists can observe the physical characteristics of tumors, track the distribution of drugs, and evaluate the effectiveness of treatment in real-time. Nanoparticle-based medicines can be customized for each patient by considering their specific tumour features, genetic profiles, and treatment histories. The individualized strategy shows potential for enhancing treatment results and surmounting drug resistance in cancer patients.
The main purpose of this work is
- To explore the potential of nanoparticle-based therapies in targeting and treating lymphatic metastasis, a dangerous characteristic of breast cancer.
- To investigate the effectiveness of nanoparticle technology in delivering drugs directly to cancerous tumors, addressing challenges in conventional delivery methods.
- To discuss the importance of nanoparticle-enabled imaging in visualizing tumors and evaluate their role in preventing post-surgical metastases.
- To explore the advance understanding and potential treatments for breast cancer progression and its associated complications.
2. LYMPHATIC METASTASIS
The most important indicator of a patient's likelihood of surviving breast cancer is lymphatic metastasis. Lymphangiogenesis, supported by circulating lymphatic endothelial progenitor cells, is the process of developing new lymphatic vessels from pre-existing lymphatics. It makes it easier for tumor cells to enter lymph nodes. The tumor cells must avoid the host response in order to survive early in a distant organ. Angiogenesis is essential for the survival and spread of metastatic foci larger than 1 mm in diameter after the initial cell transformation and growth. Proangiogenic and antiangiogenic signaling pathways combine to regulate angiogenesis. Vascular endothelial growth factor (VEGF), a proangiogenic molecule that is also activated by hypoxia, is induced in tumor cells by hypoxia. The VEGF secreted has the following effects:
- Stimulating the development of blood vessels helps newly created arteries survive in cancerous lesions.
VEGF uses its heparin-binding domain to bind to heparan sulphate proteoglycans when it diffuses to nearby blood vessels that are already there. The ECM breaks down and releases more VEGF when VEGF attaches to and activates VEGFR-2 in endothelial cells. Following this, the epithelial cells divide, move in the direction of the VEGF gradient, and eventually form tubes [6]. A specific protein known as Vascular Endothelial Growth Factor Receptor-3 (VEGFR-3) causes Lymphangiogenesis. Recent research suggests that increasing fluid load may facilitate lymphatic endothelial cell expansion, phosphorylation of VEGFR-3, and lymphatic channel development [7]. Vascular Endothelial Growth Factors (VEGFs) C and D are necessary for this factor to be activated. These lymphangiogenic elements frequently provide an environment that is favorable for the growth of new lymphatic capillaries in malignant, stromal, and tumor-infiltrating cells [8]. Clinical trials for treatments that disrupt the VEGF-C/VEGF-D/VEGFR-3 pathway have begun, and these treatments may also help some tumor types by reducing blood vessel angiogenesis.
Fig. (1) details how VEGF-1 and VEGF-2 help in the process of angiogenesis with the assistance of VEGF-3. It also describes how VEGF-3 is solely responsible for Lymphangiogenesis. Extracellular matrix, blood lymphatic vessels, immune cells, stromal cells, RNAs, numerous cellular secretory factors, proteins, and other chemicals make up the complex tumor microenvironment. It supports the development of tumor cells and is crucial for the development, progression, metastasis, and recurrence of tumors. Due to the complexity of tumor cells, the relevant pathways, the microenvironment, and their interactions, it is difficult to provide a comprehensive diagnosis and develop a targeted therapy that successfully prevents metastasis [9]. According to current theories, CSCs are found in a niche within the TME, a unique compartment that regulates CSC fate through cell-to-cell interactions or cues from the environment [10]. According to several studies, platelets are the main source of TGF- both outside of the tumor and in it. By restricting the killing capabilities of cytotoxic T cells, intratumoral TGF- may significantly reduce tumor immune sensitivity. As a result, platelet blockage may be a useful strategy for boosting immunity by preventing TGF- from reaching its source.
Furthermore, tumor metastasis brought on by spreading tumor cells is a fatal condition. A growing body of research indicates that the interaction between tumor cells and platelets is a key factor in promoting tumor spread. Platelet activation is mediated by tumor cells. In order to promote tumor growth and initiate the Epithelial-Mesenchymal Transition (EMT), activated platelets extravasate into the tumor microenvironment. This enhances the migratory and invasive capabilities of the tumor cells. Once tumor cells have entered the bloodstream, platelets protect them by acting as a barrier to immune cells, promoting tumor cell adhesion and facilitating tumor cell transmigration and metastasis [11].
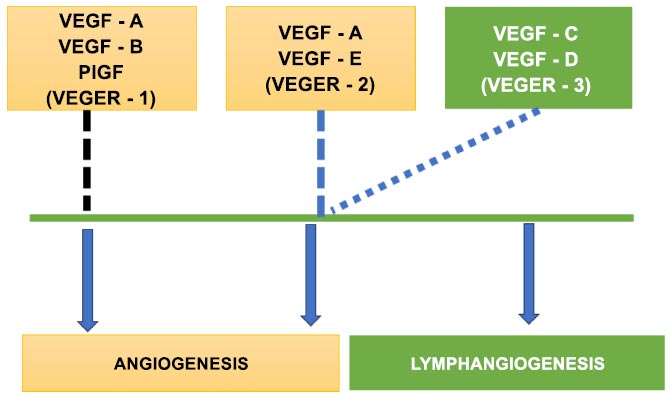
The process of angiogenesis.
In contrast to healthy tissues, solid tumors experience unregulated angiogenesis, which gives the tumor the vascular it requires to support the growing cancer cells' metabolic needs. A discontinuous endothelial-cell lining with 200–2000 nm-wide gaps between individual endothelial cells lines the resultant blood vessels [12]. Cancer-Associated Fibroblasts (CAFs) are the most common stromal cells in breast cancer patients’ tumor microenvironments, and they play a major role in disease progression through a variety of processes in close proximity to and contact with malignant cells [13]. By influencing the TME, MMPs can affect growth factors like Vascular Endothelial Growth Factor (VEGF) substances, which may encourage breast cancer growth, angiogenesis, invasion, and metastasis.
Biffi et al. [14] described the process of tumor formation after the Cancer-Associated Fibroblasts are activated by ligands secreted by the cancer cells. This leads to the deposition of the Extra Cellular Matrix, allowing for tumor formation. Collagen, fibronectin, laminin, proteoglycan, and other molecules comprise the Extracellular Matrices (ECM), a complex molecular network. ECM comes in a variety of physical and biological forms. As the connective tissue surrounding the tumor and stromal cells, the ECM regulates the growth and differentiation of breast cancer. Thus, modifying the ECM can have a profound effect on the composition and functionality of tumor tissue. As connective or fibrous tissues proliferate, the ECM stiffens, and its collagen composition rises. Collagen fibers linearly thicken due to deposition and cross-linking. The ECM's stiffness facilitates breast cancer's associated proliferation and invasion, as well as tumor cell growth, invasion, angiogenesis, and infiltration. Breast cancer progresses and metastasizes more quickly due to the ECM degradation assisted by MMPs that are overexpressed at the tumor site.
Splitting and sprouting are essentially the two perfectly timed pathways that make up the angiogenic process. Excessive microvascular stress results in intraluminal splitting into two capillaries, but tissue hypoxia more frequently triggers cell sprouting, which results in the development of a new linear capillary from an existing one. Fundamentally, “tip cells” that are found inside capillaries grow and travel to the tips of arteries during the stages of neovascularization are where angiogenesis starts. Endothelial cells with vascularization surround the pericytes and vascular smooth muscle cells of mural cells to aid in the formation of new vessel walls. Based on the tissues' needs for oxygen and nutrients, pro- and antiapoptotic factors coordinate all of these activities in an amazing manner [15].
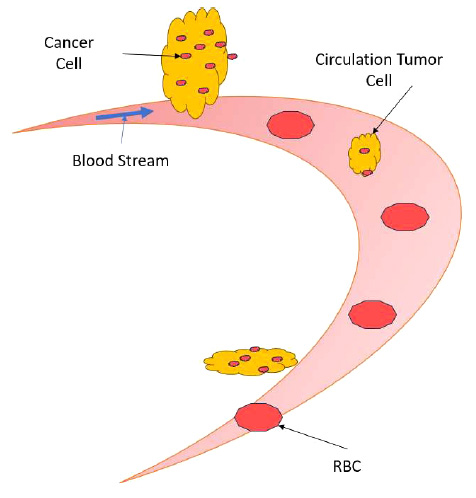
Circulation of tumor cells detach from the cancer foci.
Marta et al. [16] described how a normal breast tissue manifests itself to form a tumor as a result of homeostatic instability, later creating a pre-metastatic niche, thus paving the way for breast metastasis. One of the key causes of metastasis is circulating tumor cells. Current chemotherapy regimens are not well suited for the goal of CTC eradication. However, it is concerning that its failure might have a significant effect on breast cancer's propensity to spread and come back after surgery. To improve drug concentration at the tumor site, eliminate CTCs, and drastically reduce side effects, novel delivery tactics and procedures are required for chemotherapy for breast cancer to be more effective.
The circulation tumor cells detach from the cancer foci (which can be a primary or secondary tumor) and travel through the blood vasculature to begin a new cancer tumor site, is detailed in Fig. (2). The circulation of tumor cells detached from the primary cancer site, known as Circulating Tumor Cells (CTCs), plays a crucial role in cancer metastasis. When cancer cells acquire the ability to invade blood vessels or lymphatic channels, they can enter the bloodstream or lymphatic system and travel to distant organs, where they establish secondary tumors. This process, termed metastasis, is responsible for the majority of cancer-related deaths. CTCs are shed from the primary tumor into circulation, where they face various challenges, including shear forces, immune surveillance, and anoikis (programmed cell death triggered by detachment from the extracellular matrix). Despite these obstacles, a small subset of CTCs can survive and extravasate into distant tissues, initiating the formation of metastatic lesions. Understanding the mechanisms underlying CTC dissemination and survival in circulation is crucial for developing strategies to prevent metastasis and improve cancer outcomes. Emerging technologies such as liquid biopsy techniques enable the detection and characteri- zation of CTCs, providing valuable insights into their biology and potential therapeutic targets. Targeting CTCs and their associated pathways may offer promising avenues for intervention to impede metastatic spread and improve patient prognosis. These circulating tumor cells travel along the bloodstream, accompanying red blood cells and white blood cells.
Utilizing a dynamic light scattering particle size detector (Nano-ZS 90, Malvern Instruments, UK), the particle size distribution of the Dox micelles was determined. The morphology of these Dox micelles was examined using a Transmission Electron Microscope (TEM, H-6009IV, Hitachi, Japan) [17]. 4T1 cells were sown in 96-well plates, allowed to grow for 24 hours, and then subjected to a series of Dox micelles, free Dox, or MPEG-PCL copolymer to test for cytotoxicity. The MTT method was used to assess the health of the treated cells 48 hours after the administration of the medications. Based on the results of six different research, the average cell survival rate compared to untreated cells was calculated.
3. TARGETING METASTATIC CANCER
Traditional treatments for metastasis typically involve a combination of surgery, chemotherapy, radiation therapy, targeted therapy, and immunotherapy. Surgery aims to remove the primary tumor and any metastatic lesions that are operable. Chemotherapy involves the administration of drugs to kill cancer cells throughout the body, but it often leads to side effects such as nausea, hair loss, and increased risk of infections due to its impact on healthy cells. Radiation therapy uses high-energy rays to destroy cancer cells and shrink tumors, but it can also cause side effects like fatigue and skin irritation. Targeted therapy focuses on specific molecular targets involved in cancer growth and progression, potentially offering more precise treatment with fewer side effects compared to traditional chemotherapy. Immunotherapy boosts the body's immune system to recognize and attack cancer cells, but it can lead to immune-related adverse effects such as inflammation of organs. Despite advancements in these treatments, challenges remain in terms of reducing side effects and improving overall efficacy. Future significant advancement in recent times may involve developing more targeted therapy, chemodynamic therapy, stem cell therapy, radionics, nanoparticles, and personalized treatment approaches based on genetic profiling, and enhancing immunotherapy strategies to overcome tumor resistance mechanisms better.
Targeting cancer in the metastatic stage can be subdivided into two steps:
- Primary targeting: the affected organ, as well as all the organs affected by metastasis
- Secondary targeting: the delivered materials primarily target the cancer cells and even go into the subcellular location within the cancer cell.
3.1. Primary Targeting
The surface charge, particle size, mechanical characteristics, and chemical makeup of the nanoparticles themselves all play a role in localizing to a particular target organ. Due to these reasons, the brain is regarded as the most difficult organ to target. Some of the most effective techniques for preventing tumor spread in the context of breast cancer are
- Targeting the cancer stem cells
- Apoptosis of tumor cells
- Hindering EMT
- Modulating the TME
- Stimulating immune responses [18]
3.1.1. Chemotherapy
Chemotherapeutic drugs are often used to treat primary tumors first, but as our understanding of their molecular mechanisms has improved, we can now employ them to treat metastatic neoplasms as well. To treat cancer metastasis, numerous chemotherapeutic drugs that target various pathways involved in cancer cell survival and proliferation have been created. These therapies are broadly grouped according to how they influence tumor cell, like invasion, extravasation, angiogenesis, and tumor growth. Clinical studies have shown that the PFS and OS of patients with metastatic illness are improved by these medications. They are not regarded as selective agents because they also have a broad anticancer activity that targets numerous oncogenic pathways. For instance, patients with metastatic testicular cancer and those with ovarian cancer who have not responded to past chemotherapy treatments are treated with the alkylating drug cisplatin. The FDA-approved nucleoside analog medication gemcitabine is being evaluated in a Phase II clinical trial (NCT01028495) for the treatment of metastatic pancreatic cancer. The FDA approved eribulin mesylate for the treatment of patients with metastatic liposarcoma after a clinical study (NCT01327885) showed that it raised the median OS to 15.6 months. This non-taxane microtubule dynamics inhibitor results in the stoppage of tumor cell mitosis. A single therapy can eradicate the drug-sensitive cancer cells, but a tumor often retains a higher percentage of drug-resistant cancer cells. Combination chemotherapies, which use a number of medications, have been researched and approved by the FDA for the treatment of metastatic cancer in order to counteract this resistance.
3.2. Secondary Targeting
A specific cancer cell type is targeted precisely in this Secondary targeting. To enable the nanoparticle to bind to the cell, chemical specificity will be necessary. Nanoparticle binding with the secondary (metastatic) target differs from that with the target organ. Thus, the researcher must consider both the interest cell's immunological effects and the nanoparticle's binding affinity [19]. The pathway of treatment targeting followed after cancer is diagnosed and shown in Fig. (3).
4. NANOPARTICLE TECHNOLOGY FOR TREATMENT PLANS
With nanotechnology, the treatment plans for cancer have become very versatile. This has not only increased patient survival rate but has decreased the everlasting deadly side-effects of treating the disease.
4.1. Diagnosis
Prompt detection is pivotal for effective breast cancer management. Tumors classified as T1, with diameters under 2 cm, exhibit a 10-year survival rate of approximately 85%, contrasting with T3 tumors, which stem largely from delayed diagnosis and demonstrate a markedly lower 10-year survival rate of under 60% [20]. The initial axillary staging of breast cancer patients now routinely involves Sentinel Lymph Node Biopsy (SLNB). Since then, lymph node status has been a crucial factor in determining illness treatment strategy since it affects prognosis. After administering a radioactive colloid that is allowed to collect inside the sentinel lymph nodes, traditional SLNB is typically done surgically. This makes it easier for the surgeon to find and remove the sentinel nodes. The surgeon performs this procedure in the operating room using a clean, hand-held gamma-ray counter [21]. The sentinel nodes are taken out and put through laboratory analysis [22]. In the majority of cases, sentinel node biopsies show that breast cancer has not progressed to the axillary lymph nodes. Axillary sampling may be completely avoided in these patients if very sensitive and specific non-invasive imaging technologies could be developed to rule out the existence of metastatic illness in lymph nodes (as may be achieved with nanotechnology).
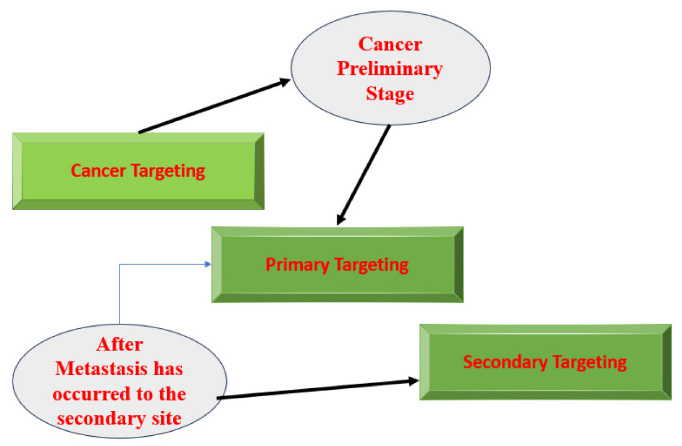
The pathway of treatment targeted.
Nanotechnology has overcome SLNB method limitations. It has further decreased morbidity associated with the evaluation of sentinel nodes in breast cancer patients. Additionally, it might enable SNLB treatments without the need for radioactive materials. Given the recent global shortages of technetium, the radioactive substance most frequently used for SLNB, this is very pertinent. Axillary surgery may no longer be necessary for many patients thanks to the potential of nanotechnology for non-invasive assessment of axillary lymph node status. Furthermore, non-invasive treatments for a metastatic illness that has spread to the axillary lymph nodes are being developed with the aid of nanotechnology [21].
4.2. Therapeutic Approaches
There are several different carriers for the mechanism employed in nanoparticle therapy. Cytotoxicity may be caused by a number of factors, including its stabilizer, structure, and zeta potential [23]. This can include biomacromolecules like siRNA or tiny molecular medicines. They can also serve as thermal absorption and imaging vehicles. The preciseness with which the medicine is released into the target region is the use of nanotechnology’s biggest benefit. This significantly reduces the likelihood of minor side effects from the cancer treatment itself. The drug’s pharmacokinetic profile is altered by the targeted release to lengthen its half-life at the sick site. Solid cancer prognostic information is mostly based on the analysis of pathological staging:
- Tumor type and size
- Invasion of lymphatic and vascular depth
- involvement of auxiliary lymph node
- Status of steroid receptor status [24, 25]
When tumor invasion and metastasis phenomena are underestimated, and antimetastatic drugs are admin- istered incorrectly, the success rate in fighting cancer is drastically reduced. The main cancer treatments are frequently based on targeted medications created to remove or shrink solid tumors, which cause cytotoxicity in cancer cells.
Once the nanoparticle is coated with the desired drug, the encapsulated nanoparticle is then stimulated to release the drug. This kind of drug delivery can take place in many ways depending on the physiological and biological microenvironment that it will be surrounded by once entering the cancer tissue. External stimuli included for this purpose are:
- Ultra-sounds
- Light
- Radio-frequency electromagnetic waves
Nanotechnology also suffices thermoablation therapy. This is the heating of affected tissues in order to kill tumor cells. The effect of this treatment can be amplified by adjusting the external stimuli.
To specifically target and treat metastatic triple-negative breast cancer, nanoparticles containing one or more therapeutic drugs can be employed [26]. The appropriateness of the nanoparticle employed for delivering drugs and genes, examining DNA architecture, etc., varies. The various types of nanoparticles that can be used include:
- Solid lipid particles
- Polymetric nanoparticles (nanospheres and nanocapsules)
- Liposomes Polymer therapeutics such as dendrimers and fullerenes
- Nanocrystals
- Inorganic nanoparticles (eg, gold and magnetic nanoparticles)
Anti-cancer medications have been effective in both in vitro and in vivo cancer therapy [27]
- Dexamethasone
- Paclitaxel
- Vincristine Sulphate (VCR)
- Curcumin
- Camptothecin (CPT)
- Doxorubicin
- Cis-platin
- Etoposide
- Rapamycin
- Fluorouracil
- Tamoxifen
The interleukin-6 cytokine superfamily, which also contains IL-6, oncostatin M, IL-11, cardiotrophin-1, ciliary neurotrophic factor, and corticotrophin-like cytokine, is made up of LIF, a glycoprotein cytokine with a molecular weight of 38 to 67 kDa. By activating several signalling pathways, the 180 amino acid protein LIF functions in several organs and cell types [28]. Arachidonic Acid (AA) is created in the body by a crucial enzyme called Delta-5-Desaturase (D5D) and an upstream -6 PUFA called Dihomo-Linolenic Acid (DGLA). When Cyclooxygenase-2 (COX-2) is overexpressed in cancer cells, breast cancer grows and spreads through a number of mechanisms. This produced AA metabolises to precancerous prostaglandins of the second series (PGE2) [29].
Dr. Shu's lab developed the first three-way junctional (3WJ) RNA nanoparticle delivery technology in a recent study. With the help of this technique, the genetic material needed for treatment is transported to the target site. Three highly affine short RNA oligomers can be used to self-assemble the nanoparticle for the 3WJ delivery technique. The resulting structure has a significantly longer half-life than bare siRNA and is particularly thermodynamically stable. It also is metabolically robust and is not easily broken down. Epidemiological studies have shown evidence that the prognosis for breast cancer may be heritable. Thus, some genetic regulation from the germline may be able to influence tumor behavior. This implies that tumor aggressiveness, occurrence, and propensity to metastasize are all heritable traits. For instance, compared to 20% of sporadic cases, between 57% to 80% of women with mutant BRCA1 genes have triple-negative phenotypic tumors. Data from various population studies did not reveal variations in breast cancer subtype incidence in cases with a family history. The results of numerous investigations have not clarified whether the characteristics of tumors are inherited. This is because the association between different types of breast cancer and events in families and close relatives is not included in this research [30].
Sabouri et al. [31] revealed that the nanocomposite created using the extract of Nymphaea alba L. leaves as a reducing and stabilizing agent has the potential to effectively treat organic pollutants and biomedical wastes produced by industrial procedures. In their study, Ghoochani et al. [32] conducted a photodynamic treatment, a well-tolerated and widely established approach for eliminating specific cancer cells. This treatment involves activating a photosensitizer agent using a specific light source. It was employed in Photodynamic Treatment (PDT) to combat MCF-7 breast cancer cells using a red light-emitting diode. Sima et al. [33] proposed that the combination of Amorphous Calcium Phosphate (ACP) and Casein Phosphopeptides (CPPs), known as CPP-ACP nanocomposite, has promising antibacterial properties for healthcare products while also promoting cell viability.
Roshanak et al. [34] suggest that chemically synthesised Silver Nanoparticles (cAgNPs) have the potential to be employed in medicine for imaging, medication delivery, and as agents against antibiotic-resistant infections, bacteria, and cancer. Andi et al. [35] conducted a study on the environmental impacts of zein nanoparticle degradation in adsorbents within the petrochemical sector. Zahra et al. [36] assess the uses of Ag-Se in photocatalytic applications and examine its cytotoxic effects. Nahid et al. [37] showed that liposomal nano-carriers loaded with curcumin have a slow-release profile and are biocompatible with the body. These nano-carriers can be used to develop drug delivery systems for hydrophobic drugs, which can be an effective strategy for treating different types of cancers. Additionally, these nano-carriers can also find applications in agriculture, pharmaceuticals, medicine, healthcare, and environmental industries. Seyedeh et al. [38] analyzed the discovery of nano-sized materials to solve numerous problems as societies advance.
The defining sites for which secondary metastases spread from the initial tumor are controlled by a process known as organ tropism. The lymph nodes, lungs, liver, bone, and brain are the organs where breast cancer spreads most frequently from this point of view. Ewing's theory was published thirty years later, which claims that the same event may be explained by the body's circulation patterns and that cells are physically stopped in the first capillary bed they encounter. The theory that cells become stopped owing to mechanical interference and/or specific chemical signals and that they then require the proper environment to start and maintain the growth of the secondary tumor can be used to explain successful metastases [39].
4.3. Medical Imaging of Breast Metastasis
Popular imaging methods used in medical imaging of breast metastases include computer tomography, positron emission tomography, near-infrared fluorescence tomo- graphy, magnetic resonance imaging, and others using active and passive targeting NPs. They contain the most thoroughly studied therapeutic medications for treating metastatic breast cancer, such as doxorubicin, paclitaxel, or docetaxel. Additionally, these particles can enhance the effects of photothermal, magnetothermal, and radiation therapy for metastatic breast cancer. Image guidance can provide useful information to strengthen therapeutic PTT regimens and ensure the safety and efficacy of photothermal ablation. The investigation of multimodal imaging for the guidance has so far included X-ray, Photoacoustic (PA), Computed Tomography (CT), and Magnetic Resonance Imaging (MRI). One significant method of cancer cell dissemination is lymphatic metastasis, and during the initial stages of metastasis, the SLNs close to the main tumor frequently serve as the host for metastatic cancer cells. With the aid of multimodal imaging support, the location of lymphatic node metastases can be identified during the full course of PTT treatment, and the therapeutic regimens can be improved. The deep tissues of the lung, liver, brain, etc. are rarely affected by distant metastases when using existing techniques, which are primarily restricted to the imaging of lymph node metastases.
Due to the integrated lymph targeting and imaging capabilities of PTN, MRI is the most widely used imaging source during PTT treatment. The regional lymph nodes close to the main tumor were easily detected using MRI and dark imaging, and the metastatic lymph nodes were successfully ablated with NIR radiation. Fluorescence imaging and MRI can be coupled for multimodal imaging-guided PTT. Because of the linked catalytic metals in SWCNT, PEGylated Single-Walled Carbon Nanotubes (SWCNT-PEG) could be employed for NIR-II fluorescence imaging (1000-1400 nm) and T2-weighted MR imaging under low-power 808 nm laser stimulation. The imaging results verified that SWCNT-PEG from the original tumor had spread to the SLNs as a result of local injection. The primary tumor and cancer cells that had spread to the SLNs could be removed by the image-guided PTT, preventing the spread of breast cancer to the distant lungs and extending survival times. HER-2 antibody-altered theranostic W18O49 nanoparticles were created for CT imaging-guided HER-2 positive breast cancer PTT. The lymph nodes in the animals with HER-2 positive metastases could be easily identified under CT guidance and selectively destroyed by NIR laser ablation, leading to a longer survival duration because of the strong X-ray attenuating and PTT potency. The metastases in SLNs may also be detected using integrated PA imaging in conjunction with folate-conjugated golden carbon nanotubes and PTT.
The ability of the RGD-NC particles to target metastasis was examined in mice with advanced 4T1 tumors (five weeks after tumor inoculation). The whole-body angiography was carried out at 99 m resolution using a micro-CT system (Siemens Inveon) and a liposomal imaging agent with a high cargo of an iodinated contrast agent. MRI imaging was used to detect metastases since it is an imaging modality that can be used therapeutically. Shortly before the medication was given, as well as 15, 30, 45, and 60 minutes later, MR images were taken. The pre- and post-injection photos used identical scanning parameters. liver scans from a metastatic animal are compared before and 45 minutes after the medication. Shows a healthy liver 45 minutes after injection to demonstrate that the liver's absorption of the drug produced a homogeneous contrast with no “hot” spots. The absolute MR signal intensity in the metastatic lesions and the healthy liver was assessed in order to objectively assess the capacity of the RGD-NC nanoparticles to target metastasis [40]. Experimental findings show that preventative care outperforms standard care, and c-HCR-based fluorescence imaging of living things can offer therapeutic direction even before clinical signs start. Lother et al. [41] have evaluated various imaging techniques, both individual and combined, such as whole-body diffusion-weighted MRI, contrast-enhanced mammo- graphy, and novel PET radiotracers specific to breast cancer epitopes, as well as exploring the role of radiogenomics in metastatic breast cancer management. Pesapane et al. [42] assessed the evidence supporting imaging modalities used to characterize metastases in breast cancer and provide an updated analysis of their comparative imaging accuracy. Comprehensive breast cancer treatment relies on thorough staging, covering local and whole-body assessments. Bone scintigraphy is favored for its ease of interpretation, wide availability, and cost-effectiveness. Emerging trends show increased utilization of whole-body MRI and hybrid imaging for distant metastasis evaluation, while PET-CT offers heightened sensitivity in metastasis detection compared to conventional imaging methods. Whole-body MRI plays a crucial role in identifying visceral and skeletal involvement, complementing other imaging modalities for comprehensive assessment.
4.4. Types of Nanoparticles
The general characteristics to determine an efficient nanoparticle are physicochemical characterization, association efficiency cell culture conditions, and flow cytometry [43].
4.4.1. Quantum Dots
Nanocrystals called quantum dots are inorganic fluorophores. It usually has a diameter of 2-10 nm. In immunoassays, immunohistochemical staining, and cellular imaging, they serve as labels. They are effective for multiplex diagnostics because numerous QDs may be activated by a single laser. Fig. (4) shows how a quantum dot is turned on and off for the purpose of drug release at the target site.
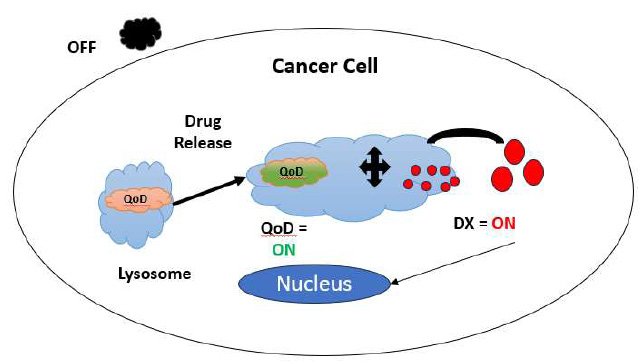
Process flow during drug release.
After a locoregional treatment, the general diagnostic technique does not enable the prediction of metastatic relapse. The fundamental factor leading to this relapse is the existence of often uncommon disseminated tumor cells and Circulating Tumor Cells (CTC), also known as Micrometastases (MMs), which are the intermediary pathogenetic link between the initial tumor and metastases in all solid malignancies. Micrometastasis is described by Huvos et al. [44] as “metastatic foci smaller than 2 mm in diameter” [45]. More than 30% of breast cancer patients lacked histological or even clinical evidence of MMs. Due to the limited prevalence of MMs, very sensitive assays based on the genetic or antigenic features of the cells generating MMs must be used. The main diagnostic methods used in this sector are immunocytochemical and molecular. Quantum dots have exceptional and distinctive optical and physico-chemical capabilities in comparison to conventional organic dyes (which are photobleachable). Quantum dots are typically attached to recognition molecules like antibodies, aptamers, or peptides to ensure cell specificity [46]. With a standard epifluorescent microscope setup, they may be seen as single nanocrystals and are significantly brighter fluorescent probes than organic fluorophores. This makes them a valuable asset for the effectiveness of low-abundance target detection.
With regard to TNBC, photothermal therapy (PTT) is a cutting-edge and quickly evolving cancer treatment strategy. Compared to the traditional cancer ablation procedures, it has good spatial selectivity and low toxicity [47]. The release of tumor-associated antigens occurs concurrently with Immunogenic Cell Death (ICD), which is induced by PTT [48]. This therapy destroys cancer cells by infusing the body with compounds that have a high photothermal conversion efficiency, using targeted identification techniques to gather around the tumor tissue, and converting light energy into heat energy by absorbing Near-Infrared (NIR) light. An innovative two-dimensional layered inorganic substance called black phosphorus has amazing qualities like:
- Strong biocompatibility.
- High light absorption
- Excellent photothermal conversion efficiency
Phototherapy has distinct advantages over other new cancer treatments, including remote spatiotemporal control, little invasiveness, and effective tumor eradi- cation. In particular, phototherapy can inhibit tumor cell migration and metastasis by reducing the adhesion force and biomechanical characteristics of the tumor cells. However, well-designed phototherapy nanoplatforms could serve as imaging and therapeutic agents, and enhanced phototherapy could improve the effectiveness of cancer treatments. For instance, photothermal agents might enable simultaneous Photoacoustic (PA) and Infrared Thermal (IRT) imaging, offering diagnosis assistance and monitoring of the efficacy of treatment. Therefore, it is predicted that creating therapeutic agents in the form of smart nanoplatforms aimed at hypoxia metastasis control and enhancing phototherapy efficacy might potentially be used to treat metastatic disorders [49].
Japanese physician Dr. J. Kurebayashi [50] provided the SCID mice with the human KPL-4 breast cancer cell line, which did not metastasize. The KPL cells were transformed into metastatic cancer cells using the pLNCX2 retroviral vector system, which carries the PAR1-GFP gene as the insert. After that, the cells were cloned to create a vast number of identical cells. It was grown in KPL and PAR1-KPL cells using a modified Dulbecco's Eagle's medium (Invitrogen). This medium contained 10% fetal bovine serum. In the presence of 400 g/ml G418 the PAR1-KPL cells were generated. The following optical apparatus was used to observe the fluorescence of GFP or QDs:
- Epifluorescence microscope
- Nipkow disc-type confocal unit
- charge-coupled device camera
An aluminum stage was created for the investigation and mounted to the microscope to eliminate oscillations caused by the mice's breathing and heartbeats. This was because it was in vivo.
This substance is used to make black phosphorus quantum dots (BPQDs), which have rapid clearing NIR photothermal effects on cancer cells. Despite having been shown to be more effective, BPQDs still have some drawbacks, including instability and poor targeting. The BPQDs can be made more stable, prevented from being recognized and cleared by immune cells, and made to concentrate and colonise effectively at the tumor site by coating them with a similar cancer cell membrane. The inspiration for this method came from adhesion molecules found on the membranes of cancer cells in recent investigations. After intradermal injections of QDs, the axillary SLN is discovered rapidly in mouse models and remains there for more than 24 hours before moving on to higher echelon nodes in the lymphatic system.
According to toxicological and biodistribution studies, QDs are largely concentrated at the injection site and within the SLN and are not renally eliminated. Initially, it was thought that QDs would eventually concentrate in the liver and spleen, among other organs, following dorsal flank injection. They don't appear to travel to other body organs when injected into a mouse paw, which more closely fits the anatomy for a breast sentinel node biopsy. Fluorescence, like silica nanoparticles, can only be seen in the SLN after the axillary skin has been removed. The biggest obstacle to the otherwise perfect nanoparticle being used in medicine is removing the heavy metal's toxicity while retaining particle stability. This obstacle might be overcome by using Near-Infrared (NIR) emitting QDs that more thoroughly enter tissue. The large variety of nanoprobes on the market includes semiconductor Quantum Dots (QDs), which have exceptional optical qualities and are gaining popularity for biomedical research applications like cancer marker detection and tumour cell imaging.
4.4.2. Super Magnetic Nanoparticles
These nanoparticles consist of magnetic materials such as iron, nickel, cobalt, and alloys of magnetic materials. They exhibit the phenomenon of supermagnetism and thermal energy. Various synthesis methods, such as chemical and physical approaches, can be employed to create these nanoparticles with controllable sizes, allowing for comparisons to biological entities ranging from cells (10–100μm) to viruses, genes, and proteins (3–50 nm). The optimization of nanoparticle character- istics, including size, distribution, agglomeration, coating, and shape, in conjunction with their distinctive magnetic properties, has facilitated their widespread application across diverse fields [51]. Magnetic Nanoparticles (MNPs) have been utilized in cancer detection, including the localization of sentinel nodes and molecular imaging [52]. Focused on their conjugation with receptor-binding ligands, magnetic nanoparticles have been employed to localize breast tumors on imaging. MNPs have the potential to induce localized tumor hyperthermia, exploiting the greater sensitivity of tumor cells to temperature elevation compared to healthy cells. Upon delivery of MNPs to the tumor site, they can release thermal energy when exposed to an alternating magnetic field, leading to the destruction of cancerous cells [53]. Superparamagnetic and biocompatible nanoparticles are directly administered into tumor tissue, where they can be manipulated by an external magnetic field to produce heat through Brownian and Neel relaxation processes [54]. Imaging lymph nodes has become the primary focus of Superparamagnetic nanoparticles (SPIOs). When an external magnetic field is applied, these particles develop inborn paramagnetic properties, preventing the undesirable phenomenon known as magnetic agglo- meration. After being administered intravenously, SPIOs travel to the lymphatic tissues where they have negative effects on T2 and T2-weighted MRI methods. This appears dark on the MRI screen. A metastatic node can be found because of the non-homogeneous uptake of contrast in the SLN.
People have utilized ultra-tiny superparamagnetic iron oxide nanoparticles (USPIOs, 50 nm) as an intravenous MRI contrast agent to evaluate the axilla. 24 hours after the injection, the afflicted axillary lymph nodes can be readily seen on a 1.5 T MRI scanner. Between 82% and 100% of patients with breast cancer are susceptible to the combo. Following the treatment of USPIOs, Koh found three patterns of lymph nodes on MRI. Gadolinium and IV USPIO injections were followed by an MRI-based axillary staging [55]. A wonderful phenomenon that resembles natural occurrences is the construction of micro-/nano-materials driven by magnetism into organized and dynamic formations. Magnetic assembly is turning out to be a fascinating subject in chemical and material science because of its stimuli-responsive and adjustable characteristics. For instance, the self-assembly of magnetic Nanoparticles (NPs) into microstructures has been used to create both soft and rigid magnetic microstructures as well as intelligent robots. Although many one-, two-, and three-dimensional (1D, 2D, and 3D, respectively) magnetic assemblies have been created under ideal physical and chemical conditions, artificial magnetic assemblies of nanocomposites in complex biological systems continue to be a major issue [56].
4.4.3. Gold Nanoparticles
Cancer cells require specific proteins and glucose to fuel their continuing growth, which allows them to divide more quickly and remain viable for longer than normal cells. The capacity of GNPs to covalently couple with diverse biomolecules via thiol groups is one of their most appealing features [57]. Near-Infrared (NIR) light absorbs and scatters from gold nanoparticles like AuNRs (gold nanorods) and AuNSs (gold nanoshells), respectively (650–900 nm). Gold nanoparticles can create heat through their own surface when exposed to EMR (electromagnetic radiation), particularly from an NIR laser. Since the peak absorbance wavelength of NIR light is in the visible range, there is negligible absorption when it passes through ordinary tissue components (450–600 nm). With relatively little harm to nearby normal tissues, gold nanoparticles activated by NIR laser illumination can cause hyperthermia in tumor tissue [58]. AuNPs can increase their uptake by cancer cells with the right functional biomolecules on top but not by adjacent normal cells (for example, the surrounding cells such as macrophages, endothelial cells, etc.). They are therefore better suited for tumor detection and treatment. Because glucose is an important source of metabolic energy for cells, cancer cells use much more of it than normal cells do. Due to the high affinity of glucose for solid cancer tumor cells, glucose-coated GNPs (or Glu-GNPs) of 20 nm in diameter are more effective at destroying solid tumors, including breast cancer. Later, the body releases the particles.
In a rat model, gold nanocages (150 nm) have been successfully used to localize the SLN following the administration of an intradermal injection. Using a photoacoustic ultrasonic transducer and a 10 Hz pulse-repetition-rate laser system, the SLN was successfully located at depths comparable to the typical depth of the axilla SLN in humans (12 mm 5 mm), spanning increasing tissue depths up to 33 mm beneath the skin's surface. Additionally, the subcapsular sinus had a dark staining from substrate deposition, which helped with ocular identification. Peak Au-nanocage accumulation was observed in the lymph node at 140 minutes after contrast injection, at quantities that were more than treble than those injected. Additionally, this investigation found no harm in the bodies of the mice. This work is currently restricted to animal models despite its success. This is due to the possibility of gold toxicity at the injection site, which could lead to an accumulation of nanocages in lymph nodes and limit clinical applicability.
Due to their excellent biocompatibility and distinctive physiochemical features, these particles are also frequently utilized as carriers for medicinal and diagnostic substances. These findings imply that through inhibiting immunosuppressive activities, AuNPs may be a promising immune modulator [59]. The IONC@Au had a significantly higher NIR absorbance than IONCs without a gold coating. IONC@Au refers to a type of hybrid nanoparticle composed of iron oxide nanocubes (IONC) coated with a layer of gold (Au). These nanoparticles combine the unique properties of both iron oxide and gold nanoparticles, offering potential applications in various fields such as biomedicine, catalysis, and sensing. The iron oxide core provides magnetic properties, which can be exploited for magnetic resonance imaging (MRI) contrast enhancement or magnetic targeting in drug delivery applications. Meanwhile, the gold shell offers surface plasmon resonance properties, making IONC@Au nanoparticles useful in photothermal therapy, biosensing, and as catalysts for chemical reactions. Overall, IONC@Au nanoparticles represent a versatile platform with combined magnetic and optical properties, enabling diverse applications in research and technology.
IONC@Au-optical PEG's and magnetic characteristics.
a) UV-Vis-NIR spectra of IONCs and IONC@Au-PEG at the same IONC concentration.
b) Curves of temperature rise for water, IONCs, and IONC@Au-PEG as seen by an infrared thermal camera using an 808 nm laser (0.5 W cm2).
c) IONCs and IONC@Au-PEG magnetization loops.
d) The relative relaxation rate and T2-weighted MR images of varying concentrations of IONC@Au- PEG particles (R2).
The effects of IONC@Au-PEG on T2-weighted MR images were evaluated using a 3T clinic MR imaging scanner, and the results showed a concentration-dependent darkening effect. The transverse reflexivity (r2) measurement, which was 83.36 mM1 S1, showed IONC@Au-PEG's potential as a T2-weighted MR contrast agent. Quantitative evaluation of the gold concentrations in SLNs, which is a relatively high level given the small diameters of lymph nodes, was used to further validate the effective MF-induced accumulation of IONC@Au-PEG nanoparticles by as much as 2.5% of the injected dose in tumor-associated SLNs on a previously described metastatic tumor model [60]. This model was made by injecting a million 4T1 murine cancer cells into the right hind foot pad.
4.4.4. Liposomes
- A class of spherical vesicles known as liposomes has an aqueous internal core and a lipid bilayer that is attached to the membrane. The lipid bilayer of liposomes, which are typically between 50 and 100 nm in size, can hold hydrophobic drugs, while the aqueous core can hold hydrophilic drugs, genes, and siRNA. Lessened systemic toxicity is just one of the advantages of liposomes.
- Low frequencies of recognition by the reticuloendothelial system and subsequent clearance
- Prolonged circulation duration
- Biocompatibility
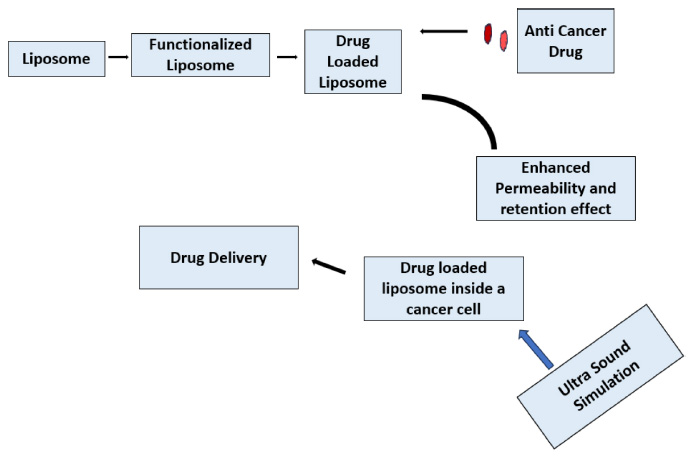
Representation of totally functional liposome.
Fig. (5) represents how a totally functional liposome is made. The liposome is first made functional, and then loaded with the anti-cancer drug. These Liposomes have an enhanced permeability and retention effect, allowing them to enter and stay in the cancer cell easily. Inside the cancer cell, the drug is delivered to do the deed [61]. Liposomes are highly efficient nanostructures with diverse formulations validated for the treatment of various diseases. These systems exhibit distinctive traits such as smaller dimensions, biodegradability, hydrophobic/ hydrophilic properties, biocompatibility, reduced toxicity, and low immunogenicity, collectively contributing to their significant efficacy in addressing different cancer types [62]. Utilizing monoclonal antibodies to functionalize liposomes represents a promising approach to enhance their specificity and mitigate the side effects of chemotherapy. Coating liposomes with an anti-HER2 monoclonal antibody enables active targeting of Human Epidermal Growth Factor Receptor 2 (HER2), which is frequently overexpressed in HER2-positive breast cancer cells [63]. Liposomes have undergone clinical exploration for various applications, ranging from imaging tumors and infection sites to delivering vaccines and gene therapies. Additionally, they have been investigated for treating infections, cancer, lung diseases, and various skin conditions, reflecting their versatility and adaptability across different medical fields [64]. Functionalization of liposomes with monoclonal antibodies targeting specific receptors, such as Human Epidermal Growth Factor Receptor 2 (HER2), enables precise targeting of breast cancer cells, potentially enhancing treatment efficacy while reducing systemic toxicity. Moreover, the ability to modify liposomal surface properties allows for controlled drug release kinetics, optimizing drug delivery to the tumor site. Liposomal formulations have been investigated in preclinical and clinical studies for their efficacy in breast cancer therapy, demonstrating promising results in terms of tumor regression and improved patient outcomes.
4.4.5. Nanodiamonds
Nanodiamonds, consisting of carbon atoms arranged in a diamond lattice structure at the nanoscale, possess exceptional physical and chemical properties. These nanoparticles exhibit high surface reactivity, bio- compatibility, and stability due to their small size and large surface area, rendering them suitable for various applications in nanotechnology, biomedicine, and materials science. Furthermore, nanodiamonds can be easily functionalized with biomolecules, polymers, or other nanoparticles to tailor their properties for specific applications. In materials science, nanodiamonds are employed to develop advanced composites, coatings, lubricants, and electronics due to their exceptional mechanical, thermal, and electrical properties [65]. Gene therapy shows immense potential for treating various diseases, including inherited disorders, acquired conditions, and cancers, but effective and safe gene delivery methods have been challenging to develop. Functional Nanodiamonds (NDs) are emerging as promising carriers for advanced therapeutics, offering high transfection efficiency comparable to conventional methods but with reduced cytotoxicity. By surface-immobilizing NDs with 800 Da polyethyleneimine (PEI800) and covalently conjugating them with amine groups, Zhang et al. [66] generated a versatile platform for in vitro gene delivery, demonstrating efficient and safe transfection properties.
Female BALB/c mice that were five weeks old and 200 and 200 20-g adult Sprague-Dawley (SD) rats that were kept in an animal care facility with a 12-hour light/dark cycle were both obtained from the Shanghai Experimental Animal Centre (Shanghai, China). The animals were acclimated for at least five days prior to the trial, and they were given fresh food every day along with free access to water.
The development of the DNX medication delivery system for the reduction of breast cancer lung metastases. High drug loading, longer circulation duration, enhanced accumulation in tumor-metastasized lungs, and improved DOX transport into tumor cell nucleus were only a few of the alluring characteristics that DNX exhibited. Due to its strong anti-metastasis efficacy and low systemic toxicity, DNX may be a feasible drug delivery approach for treating breast cancer lung metastases (lung mets) [67].
4.5. Drug Delivery
Though nanoparticle technology may seem like a breakthrough in the faster identification of new tumors through metastasis, they are unreliable. Generally, the delivery of anti-cancer drugs happens actively or passively. Passive targeting, which benefits from the fixed size of nanoparticles, takes advantage of the increased Permeability and Retention (EPR) effect as well as other particular anatomical and pathological abnormalities of the tumor vasculature. Due to the tumor's weak architecture, therapeutic nanoparticles circulated in the blood can access the tumor vasculature. The nano dimensions of the drug carriers must be smaller than the diameter of the pores in the vasculature in order to enter the tumor. Particles smaller than 150 nm have been demonstrated in experimental tests to improve cancer penetration. Nanoparticles are either absorbed by cells or entrapped in the extracellular matrix after entering the tumor vasculature. Nanoparticles are often not transported away from the tumor site because tumors have insufficient lymphatic outflow. When nanoparticles gather in cancerous cells after intravenous injection, it produces the Enhanced Permeability and Retention (EPR) effect, also known as passive targeting. Due to the universal pathophysiological phenomenon and mechanism known as EPR (Enhanced Permeability and Retention), macromolecules such as albumin and other polymer-conjugated medications that are larger than a certain size (above 40 kDa) can gradually accumulate in the tumor vascularized area. Targeting anticancer chemical retention and dispersion in solid cancer tumor tissue would be much simpler as a result [68]. Some of the most important characteristics of the EPR phenomenon include, but are not limited to:
Extensive angiogenesis
- Flawed vascular design
- A malfunctioning lymphatic drainage and recuperation system for the tumors
This phenomenon substantially improves drug absorption and efficacy by bridging developments in nanotechnology and breakthroughs in our understanding of tumor vascular biology. The majority of solid tumors have high amounts of vascular permeability factors like bradykinin, nitric oxide (a gas molecule that may block platelet activity [69]), and peroxynitrite.
Solid, quickly expanding tumors are harmed by a deficiency in nutrition and oxygen. A high vascular density is produced by the considerable angiogenesis that arises from the insufficiency. Large gaps in the endothelium cells that result from the development of tumor blood vessels cause hyperpermeability and result in a faulty architecture of the vascular endothelium. The size of typical size of the nanocarrier would be < 200 nm with hydrophilic surfaces, and the preferred weight would be > 40 kDa. Nanocarriers' targeted effect is achieved while they are still in the bloodstream. The nanocarriers often release the drug around the tumor cells once they reach the target areas. Because there is no lymphatic drainage around the tumors, the nanocarriers are retained, which eventually causes high concentrations of the anti-cancer medication to build up at the tumor site. When macromolecular substances congeal in the tumor vasculature, as referred to by anti-cancer nanoparticles, they can be maintained at the target site. The yellow particles are the anti-cancer drug loaded nanoparticles. They pass through the blood vasculature, pass through the endothelial cells, and reach the target cancer site.
5. NANOPARTICLES AS BIOMARKERS
Nanoparticles can be used for the qualitative and quantitative in vitro detection of tumor cells. By concentrating and protecting a marker from deterioration, they help the detection process to render the analysis more delicate. Liron et al. [55] conducted a study on the ways of nanoparticles as biomarkers. Understanding the potential of gold particles that have been coupled with antibodies was one method for illuminating cancer cells in an optical imaging system based on reflection. The encapsulation of inorganic biomarkers was another strategy. Since these chemicals are more photostable (compared to organic compounds) and are not hampered by the inherent fluorescence (background signal) released by cells and tissues, they are more suitable and sensitive for qualitative and, in particular, quantitative detection.
A single-ligand nanoparticle cannot be the cause of the targetable biomarkers' ever-changing expression over time and space because of how dynamic and evolving cancer cells are. A multi-ligand nanoparticle was made by combining four different ligand types on a single nanoparticle. The endothelium-based biomarkers linked to metastatic disease were its intended focus. The fibronectin, v3 integrin, P-selectin, and EGFR were some of these vascular targets. The targeting efficiency of the multi-ligand nanoparticle variations was compared to that of the single-ligand nanoparticle versions using terminal and in vivo imaging methods and studies. Using the 4T1 mouse model of breast cancer, it was discovered that multi-ligands performed more effectively than single-ligands. With roughly 2.5% of the injected dose being deposited into metastasis, it was found that all four single-ligand nanoparticle versions significantly targeted lung metastasis. A dual-ligand nanoparticle produced a deposition into lung metastases nearly two times larger than its single-ligand counterparts. The multi-ligand nanoparticle significantly outperformed its targeted nanoparticle rivals, depositing roughly 7% of its injected nanoparticles into lung metastases. Using the high sensitivity of radionuclide imaging, PET imaging showed that a multi-ligand nanoparticle labelled with fluoride was able to precisely target metastatic sickness at its extremely early stage of development [70].
6. CANCER STEM CELLS AND METASTASIS
A minor subset of tumor cells called Cancer Stem Cells (CSCs) are in charge of the development, growth, resistance to treatment, and metastasis of the initial tumor. These cells can self-replicate, quiescence, and differentiation into full tumor cells. CSC was originally noted in the context of pancreatic cancer in 2007. Additionally, during the process, pathways like Wnt2, sHh, and Notch that are in charge of CSC self-renewal were found.
These stem cells are known as BCSCs when they are used to treat breast cancer. They can regenerate themselves and go through differentiation to form a new tumor in a different organ or tissue. It is impossible to pinpoint a single factor or pathway as the precise method and process by which BCSCs arise within a tumor. After more than one likely occurrence in non-cancerous breast tissue, such as the following:
- Mutation of healthy stem cells;
- Mutation of progenitor cells;
- Mutation of differentiated non-cancerous breast cells after de novo BCSC generation;
- Microenvironmental stimulus that triggered a malignant transformation of breast cancer tissue.
The BCSCs can be identified using certain cell surface markers, including CD44, CD24, ALDH1, CD133, CD49f, and CD90.
Fig. (6) represents the four types of pathways that a normal stem cell has to take to form a breast cancer stem cell. Pathway 1: A normal stem cell undergoes mutation to form a mutated stem cell, which undergoes another form of mutation to form BCSCs. Pathway 2: The normal stem cell transforms into a progenitor cell and then mutates into a mutated progenitor cell, which later forms BCSCs. Pathway 3: The progenitor cell that is formed differentiates into breast cancer tissue, which later generates itself into BCSCs. Pathway 4: The differentiated breast tissue undergoes mutation to form a breast cancer cell, which in turn forms BCSCs.
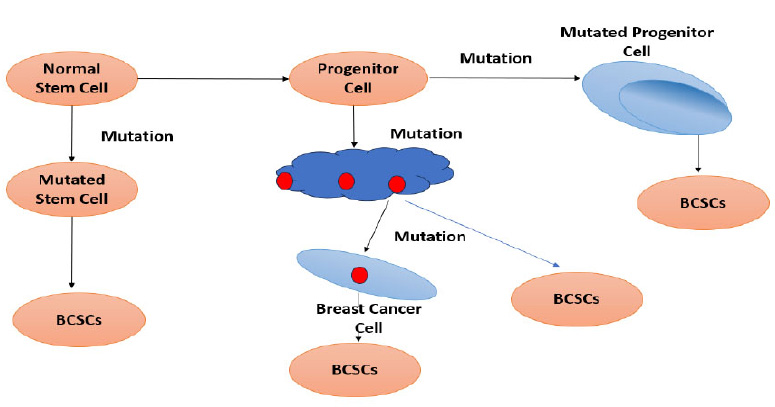
Types of pathways from normal stem cells to form a breast cancer stem cell.
During adolescence, pregnancy, and lactation, the mammary gland goes through a lot of remodeling, differentiation, and development. This dynamic development's foundation shows that mammary stem cells serve as the foundation for the potential for regeneration. Numerous investigations have demonstrated that normal breast tissue contains stem-like cells. They can self-renew and differentiate into myoepithelial, ductal, and alveolar epithelial cells. These cells are undifferentiated. Additionally, mammary stem cells showed a large overlap in gene expression with embryonic, neuronal, and hematopoietic stem cells [71]. The Cancer Stem Cell (CSC) hypothesis postulates that a specific subpopulation of CSCs may be hierarchically arranged and in charge of upholding a variety of solid tumor forms. According to the cancer stem cell paradigm, cancer stem cells are a source of self-renewing cells inside the tumor that supply the diverse lineages of cancer cells that make up the tumor. The terms “tumorigenic cell” or “tumor starting cell” are also used in literature. It is well recognized that only a tiny subset of disseminated tumor cells are capable of metastatic colonization and that only a small percentage of cancer cells can develop tumors [72]. It is important to target Cancer Stem Cell signaling pathways. Table 1 shows the functions and their activations along with the results obtained.
| Sl. No. | Pathway | Functions | Activates | Result |
|---|---|---|---|---|
| 1 | Wnt/β-catenin signaling pathway | Alters a number of physiological processes, including self-renewal, growth, regeneration, and development. | A Wnt ligand binds to the transmembrane complex that makes up the Frizzled receptor, causing it to be activated. | Induces the low-density lipoprotein-related receptor to bind, which in turn inhibits the protein that binds to glycogen synthase kinase-3, enhancing the stability of -catenin, which subsequently accumulates and is transported to the nucleus. This improves the performance of target genes. |
| 2 | Notch Signalling Pathway | Oversees a wide range of activities, including the choice of cell differentiation and the emergence of particular tissue patterns. In controlling the upkeep and differentiation of stem cells, it also has a significant impact. | According to certain theories, Notch 1 and 2 have the greatest degree of structural and sequence similarity and are found in all kinds of tissues. Notch 2 and 4 on the other hand, are expressed in a smaller variety of cell types. | Compared to the use of a single free small molecule TGF inhibitor, the therapeutic effectiveness in tumor xenograft models has been markedly increased. |
| 3 | Transforming growth factor-β signaling pathway | During the early stages of normal development and regeneration, key regulatory signals are sent, and it also promotes the onset and spread of certain cancer forms, including breast cancer. | The type II receptor that the TGF- (Transforming Growth Factor - beta) ligands bind to recruits and phosphorylates a type I receptor on a regular basis. The type I receptor then causes phosphorylation, which leads to transcription that is activated by the ligand. Additionally, compared to cancer patients with normal TGF- levels, those with elevated TGF- levels in urine and blood samples have demonstrated shorter survival times. | This made it clear how TGF- and cancer patients' worse prognoses and advanced disease states are related. TGF- signaling is a key predictive factor in a number of malignancies, according to these studies. |
| 4 | Hedgehog signaling pathway | Essential controller of pattern creation in the early stages of regeneration. Additionally, it controls migration, growth, and differentiation in a manner that depends on time, place, and concentration. The functional significance of the signaling pathway is illustrated by the rise in malignancies and birth defects connected to incorrect adult activation of this normally quiescent channel. | Smoothened's (Smo) inhibition is lifted when Hh binds to the 12-transmembrane receptor Patched 1. This triggers the Hh signaling cascade, which causes the nuclear localization of Gli transcription factors in the target cells. According to various study teams, Hh signaling is inappropriately active in a number of cancer forms, including breast cancer. | Therefore, focusing on the Hh signaling pathway may offer a potent therapeutic strategy for treating a variety of malignancies. The Gli antagonist (HPI-1)-conjugated polymeric nanoparticle (NanoHHI) was created and characterized due to the restricted therapeutic use of this route. NanoHHI significantly decreased the invasion and proliferation of CD133-positive cells, which are presumed to be CSCs in malignancies. |
Through studies and experiments, it has been seen that target-specified nanotechnology has been administered to prevent metastases. The use of nanoparticles to treat triple-negative breast cancer (TNBC) is discussed in the paper that follows. The distinctive traits of TNBC are as follows:
• Does not express the estrogen and progesterone hormone receptors.
• Human epidermal growth factor receptor 2 (HER2) deficiency
In their absence, TNBC has a poor prognosis and few treatment options. These receptors are typically used to target treatment. This is because of the overexpression of EGFR in 70 percent of the patients affected by TNBC. This is a hindrance to conventional chemotherapy.
7. POST-SURGERY PREVENTION OF METASTASIS
In recent years, the surgical treatment plan and methodology for breast cancer have changed from being the most pleasant to the least effective. It is a known fact that surgery is very often successful in removing the primary tumor for a patient. It is very fortunate that this works. But more so than ever, cancer reoccurrence occurs through metastasis. More than 90% of cancer-related deaths are caused by cancer recurrence [73]. This time, metastatic tumors go unnoticed for a long time, very often being identified in the fourth stage of cancer (final stage). The time period before reoccurrence can range anywhere from a span of months to even years. It does not necessarily have to be cancer of the previously affected organ; the primary cancer organ can be a completely new organ this time. This reoccurrence due to metastasis is mostly due to the following reasons:
- Residual microtumors
- Circulating Tumor Cells (CTC)
- Improvements in DNA damage repair
- Upregulation of proteins involved in apoptosis, such as tyrosine kinase
- Other developmental influences
Despite the improvement in surgical and other treatment techniques, the two reasons mentioned above seem to be inevitable in most cases. One such study has proven the good effect of the combination of immunotherapy with nanotechnology to create hybrid Nanovesicles (hNVs) that amplify the macrophage response against metastasis post-surgery. The constituents of hybrid nanovesicles (hNVs) are:
- Platelet-derived Nanovesicles (P-NVs)
- M1 macrophage-derived nanovesicles (M1-NVs)
- Cancer cell-derived nanovesicles overexpressing affinity SIRPα variants (SαV-C-NVs)
Since these are lipids and proteins from the source cells, the nanovesicle can inherit the source cells' various special abilities.
In order to create hNVs, there are two key phases that must be completed:
- Gathering/obtaining modified cells and the resulting nanovesicles
- Forming hNVs by fusing the separate NVs.
9. PREVENTION
Currently, techniques to improve cancer prevention are being developed using nanoscale engineering. Anti-estrogen nanoemulsion formulations have been demonstrated to be more effective at reducing breast cancer cell growth and proliferation. Nune et al. [74] recently created biocompatible gold nanoparticles by employing green tea's anti-neoplastic and immune-boosting polyphenols as gold reduction agents. Epigallocatechin-3-gallate, a green tea polyphenol, is more effective at promoting apoptosis and inhibiting angiogenesis when it is encapsulated in poly-lactic acid nanoparticles, as shown by Siddiqui et al [75]. Additionally, nanoparticles are being developed to increase the bioavailability of flavonoids and, hence, their anti-cancer capabilities. It has been shown that gold nanoparticles prevent angiogenesis by interacting with heparin-binding proteins. Gold nanoparticles have been demonstrated to inhibit angiogenesis via their interaction with heparin-binding proteins. In addition, polyhydroxyl fullerenes have been demonstrated to decrease tumor growth in mouse cancer models and to have immunostimulatory effects.
10. DISADVANTAGES OF NANOTECHNOLOGY
However, there is still a long way to go before nanoparticle technology is widely used in medicine. It involves dealing with a number of unavoidable difficulties, including toxicity, endocytosis by target cells, stability and degradation in a physiological milieu, and endosomal escape.
CONCLUSION
In conclusion, nanoparticle technology has the potential to be a promising new approach for preventing lymphatic metastasis in breast cancer. Nanoparticles can be used to deliver drugs or other agents directly to target cancer-specific tumors, where they can kill cancer cells or prevent them from spreading. It is clear that they can be used to deliver drugs or other agents that are difficult to deliver using traditional methods. Nanoparticles can also be used to image tumors, which can help doctors to better understand the extent of cancer and to plan treatment. Extended research in this sphere can be used to image tumors more accurately, which can help doctors to better understand the extent of cancer and to plan treatment. This is a very important step in biomedical engineering, as it will only increase the efficacy of therapy and reduce the side effects of the treatment plans for this monstrous disease. Though more extensive research is needed to develop and optimize nanoparticle-based therapies for breast cancer, it has the potential to revolutionize the way that breast cancer is treated.
LIST OF ABBREVIATIONS
| EMT | = Epithelial-Mesenchymal Transition |
| TME | = Tumour Microenvironment |
| QOL | = Quality of Life |
| EPR | = Enhanced Permeability and Retention |
| MRI | = Magnetic Resonance Imaging |
| CT | = Computed Tomography |
| PET | = Positron Emission Tomography |
| VEGF | = Vascular endothelial growth factor |
| VEGFR-3 | = Vascular Endothelial Growth Factor Receptor-3 |
| TNBC | = Triple-negative Breast Cancer |
| HER2 | = Human epidermal growth factor receptor 2 |
| CTC | = Circulating Tumor Cells |
| P-NVs | = Platelet-derived Nanovesicles |
| M1-NVs | = M1 Macrophage-derived Nanovesicles |
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


