All published articles of this journal are available on ScienceDirect.
The Role of Neuroimaging in the Latent Period of Blunt Traumatic Cerebrovascular Injury
Abstract
Introduction:
Blunt cerebrovascular injury (BCVI) is found in 1-2.7% of all blunt trauma when appropriate screening criteria are employed. A significant number of patients with BCVI have a latent, or asymptomatic period, in which therapeutic intervention based on the appropriate use of angiographic imaging may decrease the risk of an ischemic stroke.
Methods:
Case report and review of literature.
Results:
A 42 year old woman suffered a fall off a motorcycle and was neurologically intact in the emergency room. Fractures involving the transverse foramen of cervical vertebrae were found on non-contrast Computed Tomography (CT) but screening for BCVI with angiographic imaging not performed. She subsequently suffered an ischemic stroke resulting in significant disability. Published studies that address the use of screening criteria for BCVI and subsequent management are reviewed.
Conclusion:
BCVI results in significant morbidity and mortality attributable to ischemic stroke. There is often a latent period between BCVI and occurrence of ischemic stroke. Specific risk factors can be used to identify patients requiring screening with catheter or CT angiography. Treatment with antithrombotic agents is the mainstay of treatment of BCVI and may reduce the rate of ischemic stroke. Identification and treatment of asymptomatic BCVI in blunt trauma patients may prevent ischemic stroke in a predominantly young population.
CLINICAL SCENARIO- THE PATIENT WITH BLUNT TRAUMATIC INJURY AND NO EARLY DEFICIT
A 42 year old woman was brought to an outside facility after a fall from a motorcycle. She had a 15 minute loss of consciousness. Following this, her Glasgow Coma Scale (GCS) was 15. CT brain revealed a small amount of traumatic subarachnoid hemorrhage. Imaging revealed non-displaced fractures of the transverse processes on the right side of the second, third, fourth and seventh cervical vertebrae (C2, C3, C4 and C7) extending into the foramen transver-sarium (Fig.1). Imaging also revealed compression fractures of sixth and eighth thoracic vertebrae (T6 and T8) without impingement on the spinal canal. She was also found to have a fracture of the distal right radius, dislocation of the left elbow and a grade 2 splenic laceration on imaging. She underwent closed reduction and external fixation of the right forearm. Following the surgery, she was neurologically intact. The next day she abruptly became mute with deviation of the head and gaze to the left with right hemiparesis. She was emergently transferred to our facility for management of acute stroke. A non-contrast CT of the brain revealed loss of grey-white differentiation in a left middle cerebral artery distribution and a CT angiogram revealed dissection of both carotid arteries immediately proximal to the skull base as well as dissection of the left vertebral artery in the neck. On the left side the distal cervical internal carotid artery was found to be nearly occluded (Fig.2) with only a string sign in the carotid canal, with patency of the distal left intracranial internal carotid artery and middle cerebral artery. On the right side, a raised intimal flap with early signs of pseudoaneurysm formation was seen with >50% narrowing of the lumen of the vessel (Fig.3). The left vertebral artery demonstrated luminal narrowing of >50% just prior to entering the foramen transversarium of the sixth cervical vertebra (C6) and also demonstrated an intimal flap with narrowing of the lumen >25% at the level of the first cervical vertebra (C1). Magnetic Resonance Imaging (MRI) of the brain confirmed a large left middle cerebral artery territory infarct (Fig.4). The patient was not considered to be a candidate for anticoagulation with heparin in view of the splenic laceration and endovascular management was not performed in view of the completed infarct on the left side. She was started on Aspirin 81mg a day. She remained globally aphasic and right hemiparetic for the duration of the Intensive Care Unit (ICU) stay. The Intracranial Pressure (ICP) via CodmanTM monitor was briefly elevated to 20-30mmHg and the patient was successfully treated with osmotherapy rather than decompressive surgery. The patient was transferred out of the ICU after several days and seen in clinic follow up 3 months later at which time significant neurological improvement was seen. The patient was able to communicate and follow commands although a significant expressive aphasia remained. She was able to ambulate with assistance although she had very limited function of her right arm. A repeat CT angiogram at 3 months revealed significant healing of the dissection on the left side to <50% (Fig.5) while on the right side there was progression of the pseudoaneurysm to 18mm in length (Fig.6). The left vertebral lesions were unchanged. The patient was maintained on 81mg of Aspirin to decrease the risk of thromboembolism and referred for endovascular management in view of the progressive nature of the pseudoaneurysm.
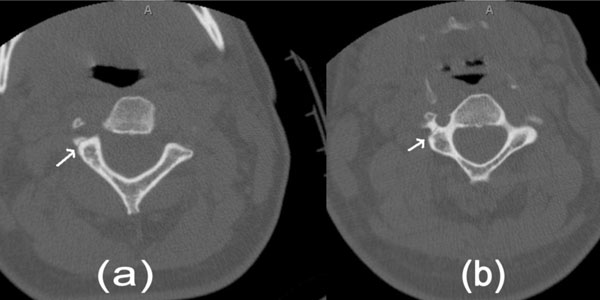
CT non-contrast of the cervical spine. a). Fracture of the right transverse process of C2 involving the transverse foramen. b). Similar fracture passing through right transverse foramen of C3.
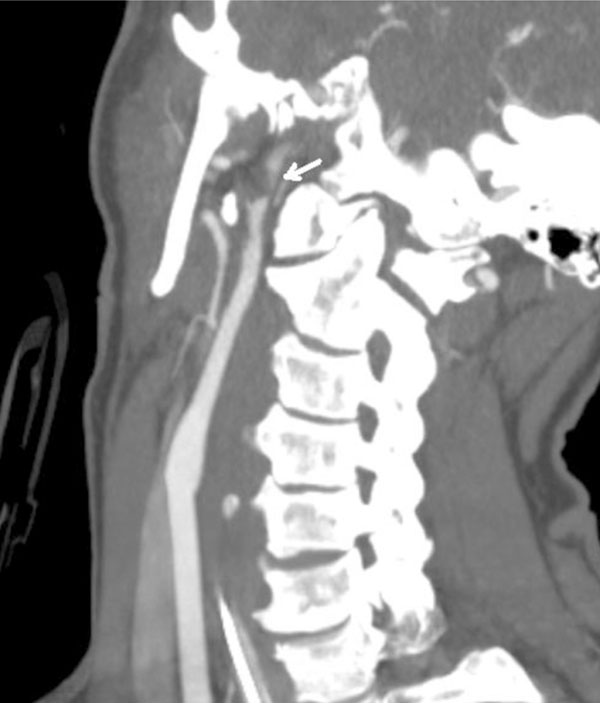
CT angiogram oblique view. Grade 2 injury of the left carotid artery with severe narrowing of the lumen proximal to the skull base. There was only a trickle of flow visualized in the petrous and cavernous segments distal to this lesion.
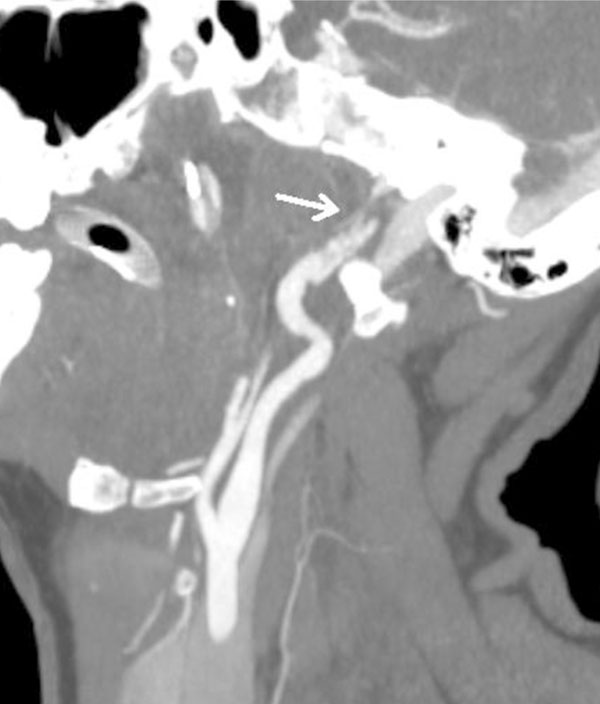
CT angiogram oblique view. Grade 2 injury of the right carotid artery proximal to the skull base with raised intimal flap.
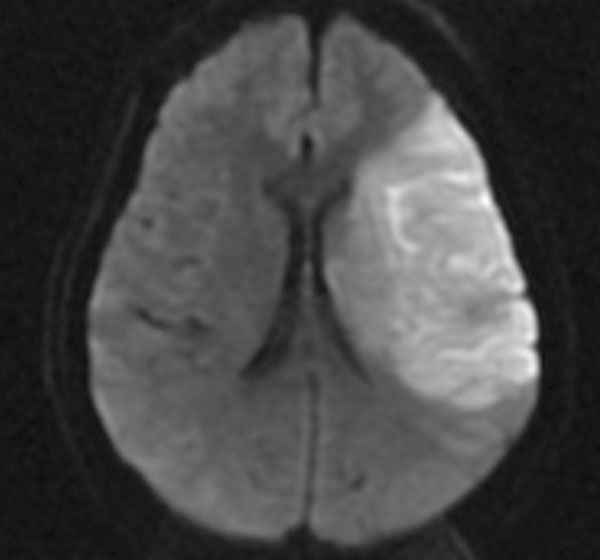
MRI of the brain with region of restricted diffusion consistent with infarct in the territory of the left middle cerebral artery.
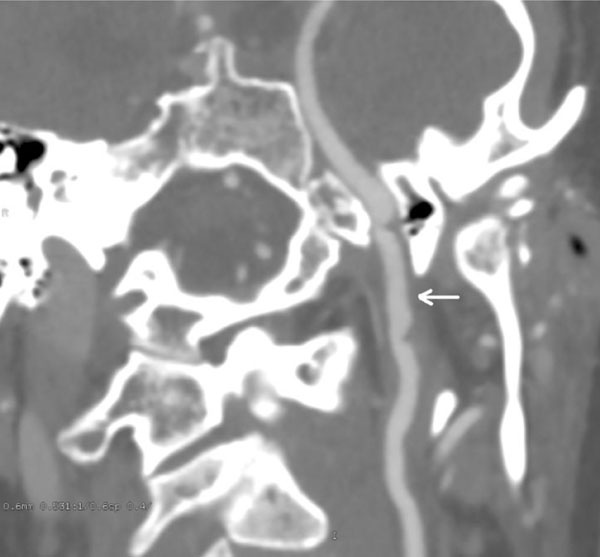
CT angiogram oblique view, at 3 month follow-up. Significant healing of the left carotid injury. There was normal flow visualized distal to the injury.
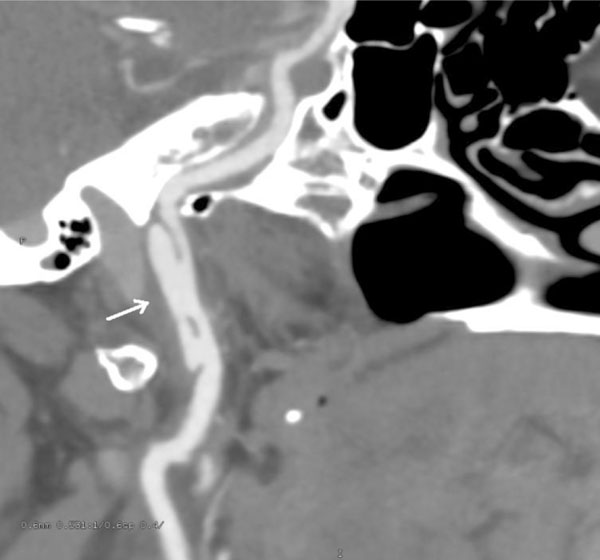
CT angiogram oblique view, at 3 month follow-up. Progression of the injury in the right carotid artery to pseudoaneurysm, 18mm in length.
Introduction
An estimated 1.5 million head injuries occur every year in the United States and Traumatic Brain Injury (TBI) is the leading cause of death and disability in children and adults from ages 1 to 44 [1]. Blunt cerebrovascular injury (BCVI), or injury to the carotid and vertebral arteries in the setting of blunt trauma, was originally reported to be very uncommon, occurring in 0.08% of patients [2]. More recent studies, however, report a prevalence ranging from 1.1-1.6% of all patients with blunt trauma [3-5], with a prevalence of 2.7% in the most severely injured (injury severity score≥16) [6]. This increased prevalence is almost certainly a consequence of screening protocols instituted at several institutions. BCVI results in significant disability and morbidity- 32-67% for carotid injury and 14-24% for vertebral injury [7-11]. While patients with BCVI often die because of other injuries (particularly severe TBI), there appears to be significant attributable mortality to BCVI itself, with one study reporting attributable mortality up to 38% [4]. The more wide-spread use of screening for BCVI using CT or catheter angiography is mostly driven by the awareness that a significant number of these injuries are initially asymptomatic, prior to presenting with symptoms of cerebral ischemia/ infarction, and that detecting these lesions when they are asymptomatic may provide an important window of opportunity to prevent ischemic strokes.
Mechanisms of Injury
The mechanism of blunt injury to the carotid appears to be through longitudinal stretch of the vessel, often against the transverse processes of C1/ C2 in the context of hyperextension and contra-lateral rotation, thereby accounting for the usual location of these injuries in the distal cervical internal carotid artery, just proximal to the skull base [12, 13]. Carotid BCVI also occurs with fractures of the skull base, particularly those involving the carotid canal. Vertebral artery injuries occur in the context of spinal subluxation/ dislocation and fractures running through the transverse foramen resulting in stretching of the otherwise fixed vertebral artery against the surrounding bony structures or dural margin [14, 15]. Other mechanisms of BCVI include direct trauma to the anterior neck (as with hanging), intraoral injuries and displacement/ fracture of the mandible. Thromboembolism likely results from platelet aggregates that form when the intima is disrupted or within pseudoaneurysms, resulting in distal embolism or luminal narrowing/occlusion and limitation or obstruction of flow. Stroke may also occur when intramural hematomas and intimal flaps cause hemodynamic compromise. Available data suggests that the majority of patients with carotid artery dissections suffer brain injury more consistent with a thromboembolic mechanism [16]. Less common mechanisms of stroke include complete transection and venous hypertension with arterio-venous fistulas (AVF).
Screening Criteria for the Use of Neuroimaging
It is clear that while some cases of BCVI present early with focal neurological signs/ symptoms or active arterial bleeding and others never become symptomatic, there is a significant group of patientswith BCVI who demonstrate a latent period, lasting from hours to days, before suffering an ischemic stroke. In different case series, 23-50% of patients with BCVI who develop symptomatic cerebral ischemia do so after a period of 12 or more hours after the injury, sometimes up to 7 days [17-20]. This latent period may be a window in which therapeutic intervention can be initiated to prevent subsequent cerebral ischemia. Knowledge of the traumatic injuries that are most commonly associated with BCVI, including asymptomatic BCVI, has led some authors to propose screening criteria based on certain clinical or radiological findings. The most widely used screening criteria for BCVI were developed in Denver, Colorado, based on the work of Biffl [9, 21] and Cothren [22] et al. The Denver criteria are listed in Table1. In addition to these criteria, the presence of major chest trauma also seems to predict the presence of BCVI [23]. It is important to note that 20-24% of patients with BCVI will not be picked up by the aforementioned screening criteria and may present with neurological signs and symptoms despite a policy of screening for BCVI [19, 21]. Interestingly, our patient described above, who was not screened for BCVI, did not have any of the classical risk factors for carotid injury (base of skull fracture, diffuse axonal injury, Le Fort 2 or 3 fracture) but did have a risk factor for vertebral artery injury (fractures passing through the transverse foramen, Fig. (1)) and therefore would have fit screening criteria for BCVI. It is useful to note here that multi-vessel injury, as seen in our patient, is common, occurring in 18-38% of BCVI and should be actively looked for when any one vessel injury is detected [18, 19, 24, 25].
Denver Screening Criteria for BCVI18
| Signs/symptoms of BCVI |
| Arterial hemorrhage from mouth, ears, nose or wound |
| Cervical bruit in a patient <50 years old |
| Expanding cervical hematoma |
| Focal neurological deficit |
| Neurologic examination incongruous with CAT scan findings |
| Ischemic stroke on secondary CAT scan |
| Risk factors for BCVI |
| High-energy transfer mechanism with |
| Lefort II or III fracture |
| Cervical spine subluxation, fractures extending intotransverse foramen, fractures of C1C3 |
| Basilar skull fracture with carotid canal involvement |
| Diffuse axonal injury with Glasgow Coma Scale score _6 |
| Near hanging with anoxic brain injury |
Diagnosis and Grading of Injury
The diagnostic tool most appropriate for screening for BCVI is a matter of debate. Four vessel digital subtraction angiography (DSA) is widely accepted as the gold standard, but does involve the small but definitive (generally <1%) risk of procedural thromboembolism and stroke, as well as minor or major bleeding into the thigh or retroperitoneum. More recently, multidetector Computed Tomographic Angiography (mdCTA) has seen widespread use as a less invasive screening tool for BCVI. The frequent need for CT scanning of other parts of the body makes this particularly expedient, with some institutions including CT angiography of the neck as part of a whole- body rapid multidetector spiral CT scan, involving one spiral data acquisition and one contrast injection for the entire body [26]. While whole-body scanning is much more frequently associated with the presence of artifacts, it does seem to detect clinically significant BCVI with accuracy comparable to focused md CTA of the neck [24]. Two studies have looked at the use of mdCTA as a screening tool for BCVI with DSA performed only to confirm positive findings on CTA [3, 27], No patients with negative mdCTAs (who did not undergo DSA and were followed clinically) suffered clinical neurological decline in either study. Two studies have directly compared 16-channel mdCTA to DSA. The earlier study performed both mdCTA and DSA in all patients who met screening criteria for BCVI [28]. The sensitivity and specificity of mdCTA compared to DSA were 98% and 100% respectively. A subsequent study that also performed mdCTA and DSA in all patients meeting screening criteria reported very different results, with sensitivity and specificity of only 74% and 84% respectively [29]. Of note, though, when only the second half of enrolled patients were analyzed, the sensitivity and specificity jumped to 100% and 86%, suggesting that the presence of a significant learning curve for radiologists in the use of mdCTA to diagnose BCVI.
Magnetic Resonance Imaging (MRI) and Magnetic Resonance Angiography (MRA) are frequently used in the context of ischemic stroke for the diagnosis of cervical artery dissection. A retrospective comparison of 18 patients with ischemic stroke associated with cervical artery dissection evaluated with both mdCTA and MRI/ MRA suggested that CTA may be superior for the depiction of dissection, particularly in the vertebral artery [30]. MRI and MRA have not been systematically evaluated for the diagnosis of BCVI. The use of MRImay, at several institutions, pose logistical problems in terms of availability, time taken for the study and the ability to monitor potentially unstable trauma patients while in the scanner. Duplex ultrasound has been used as a non-angiographic method to screen for BCVI. Carotid duplex ultrasound appears to have very poor sensitivity in comparison to 4-vessel or CT angiography. One retrospective study compared 407 trauma patients screened with mdCTA for BCVI with an earlier group of 1471 patients screened with carotid duplex sonography [31]. Carotid duplex detected five cases of BCVI but missed another eight cases associated with ischemic stroke. Sensitivity and specificity of carotid duplex were 38.5% (95% Confidence Interval [95%CI]: 13.9-68.4%) and 100% (95%CI, 99.7-100%), respectively. In contrast, CT angiography detected BCVI in 11 patients, with a sensitivity of 100% (95%CI 71.5-100%), but produced one false-positive result.
In order to standardize the reporting of BCVI, Biffl and coworkers have proposed an angiography based grading system for BCVIs (Table 2) [32]. The risk of stroke appears to be directly correlated with angiographic grade (Table 2) in carotid BCVI, while in vertebral BCVI, interestingly, the highest risk of stroke appears to be with Grade II injuries (luminal narrowing >25%, intraluminal thrombi or AVF) and least with Grade III (pseudoaneurysm) [17].
Blunt Carotid and Vertebral Arterial Injury Grading Scale32
| Injury Grade | Description | Prevalence of Stroke with Carotid Injury17 |
|---|---|---|
| I | Luminal irregularity or dissection with _25% luminal narrowing | 3% |
| II | Dissection or intramural hematoma with _25% luminalnarrowing, intraluminal thrombus, or raised intimal flap | 11% |
| III | Pseudoaneurysm | 33% |
| IV | Occlusion | 44% |
| V | Transection with free extravasation | 100% |
Treatment
Medical treatment with antithrombotic agents appears to be the mainstay of management of BCVI. A number of retrospective studies have shown a large reduction in the rate of neurological sequelae with the use of antithrombotic therapy compared to patients who, for any reason, were not treated with anticoagulant or antiplatelet therapy [8, 10, 18]. In one such study by Cothren et al., BCVI was diagnosed in 244 patients using the Denver screening criteria [11]. Of 187 patients eligible for and treated with antithrombotic therapy, one (0.5%) suffered a stroke while 21% of patients not treated (generally because of the presence because of a contraindication such as bleeding risk) suffered a stroke. Patients who suffered strokes had a significantly higher BCVI-attributable mortality (21% in carotid BCVI, 18% in vertebral BCVI) compared to those who did not suffer a stroke (0% for both carotid and vertebral BCVI). In another study by the same group, over a 7 year period in which prospective screening criteria for angiography were used, 643 patients were screened and 114 patients found to have blunt carotid injury. Of these, 73 were treated with either anticoagulation (n=56) or antithrombotic agents (n=17) and the rest were not treated, most commonly because of the presence of contraindications. None of the treated patients developed an ischemic stroke while 46% of the untreated patients suffered “ischemic neurologic events” [18]. There are no randomized controlled trials comparing anticoagulation with Heparin to the use of antiplatelet agents in BCVI. The large number of patients who would likely need to be enrolled to obtain a meaningful result makes such a trial challenging. Most studies that have performed subgroup analysis of non-randomized and retrospective studies to address this question have found no difference between the two groups [5, 18, 33]. In the absence of data from randomized controlled trials, some experts, including the Denver group, do preferentially recommend the use of Heparin in patients without major bleeding risk on the basis of some studies that have demonstrated a trend toward reduction in stroke rates with Heparin as compared to Aspirin. One study by Biffl et al. showed a 1% vs. 9% rate of stroke (p=0.07) with Heparin vs. Aspirin [34]. Serious bleeding complications can, however, complicate the use of anticoagulation. In the same study from the Denver group by Biffl et al., bleeding requiring transfusion or cessation of Heparin was seen in 54% of patients, on the basis of which the authors recommend a more conservative Partial Thromboplastin Time target of 40-50 seconds while using Heparin. Worsening of intracranial bleeding has also been reported with the use of anticoagulation to treat BCVI [35]. Our patient was treated with Aspirin instead of Heparin- despite the presence of a symptomatic near-occlusion of the left carotid and a Grade 2 injury of the right carotid- because of the presence of a concomitant splenic laceration.
No data currently exists on when antithrombotic therapy should be discontinued. Some authorities recommend making a decision based on follow-up imaging after 7-10 days [36]. Antithrombotic therapy is stopped if the injury has healed, while if persistent injury is seen, therapy with an antiplatelet or Warfarin is continued for another 3 months at which time a DSA or mdCTA is repeated.
The role of open surgery appears to be very limited in BCVI. Most carotid injuries are close to the skull base and not easily accessible. In patients with surgically accessible injuries, however, patients who do not have a dense neurological deficit who undergo reperfusion appear to do better than those who undergo ligation [37]. Patients with a dense neurological deficit, however, seem to do poorly regardless of the specific surgical treatment chosen [38].
The role of endovascular techniques in the management of BCVI is not clearly defined. Several case series have indicated the safety and feasibility of emoblization of pseudoaneurysms and stenting of intimal injuries [39-42]. One retrospective study from the Denver group, however, reported a much higher rate of carotid occlusion in patients who underwent stenting than those who underwent medical management with a consequently higher rate of symptomatic complications (21% vs. 5%) [43]. The retrospective nature of this study and the likelihood of selection bias makes it difficult to draw meaningful conclusions from this study, however, and other studies have reported very good results with endovascular procedures performed in patients with dissections. Advances in endovascular techniques and devices have made stenting and coiling of distal extracranial as well as intracranial lesions feasible. Ansari et al. have reported excellent results with the use of the neuroform stent to treat distal extracranial as well as intracranial dissections [44]. Kadkhodayan et al. reported a case series of 26 patients with carotid dissections, with or without associated pseudoaneurysm, treated with angioplasty and stenting [45]. After stenting, only one patient was left with significant vessel lumen stenosis (50%). Three patients experienced procedural TIAs, and there were no procedural strokes. One patient developed a new intimal flap, resulting in termination of the procedure. At mean follow-up of 14.6 months, two patients experienced angiographic occlusion of the treated vessel (one at 22 days and the other at 3.4 months). One was asymptomatic, while the other experienced a stroke contralateral to the treated side. Two patients experienced recurrent ipsilateral Transient Ischemic Attacks, at 2.7 months and 12 months, respectively. The use of endovascular stents in patients with multi-system trauma can be problematic because of the need for dual antiplatelet therapy, often for several months, in patients at high risk for bleeding.
Follow-Up Imaging
BCVIs are dynamic lesions and both treated and untreated injuries can either heal or progress. Most authorities recommend obtaining follow-up imaging after 7-10 days in patients with BCVI, since this appears to frequently influence management. As an example, Biffl et al. reported the results of follow up imaging at 7-10 days in 114 patients with carotid BCVI and 79 patients with vertebral BCVI [5]. 57% of grade 1 and 8% of grade 2 injuries healed, allowing cessation of antithrombotic therapy. 8% of grade 1 and 43% of grade 2 lesion demonstrated progression, requiring referral for endovascular management. Patients with grade 3 and 4 injuries, however, rarely showed much change, remaining stable 93% and 82% of the time. Interestingly, in our patient, both healing and progression was seen in different vessels, with the symptomatic left carotid near-occlusion demonstrating significant healing; and the right carotid BCVI demonstrating progression from a grade 2 to a sizeable grade 3 injury, requiring referral for endovascular management.
Efficacy of Angiographic Screening
No prospective, randomized controlled trial has validated a policy of aggressively screening blunt trauma patients for BCVI. Many non-randomized studies, including the previously mentioned studies by the Denver group, however, strongly suggest a large reduction in the risk of stroke when asymptomatic patients with BCVI are treated with antithrombotic agents [8,10,11,18]. In a more recent study by Schneidereit et al., 8-channel mdCTA was used to screen patients with prospectively identified risk factors for BCVI [4]. 170 patients were screened over a year and the prevalence of BCVI rose from 0.17% to 1.4%, while, more significantly, the BCVI related stroke rate as well as BCVI-specific mortality dropped to 0% from 67% and 38% respectively. While there may significant expenditure with the widespread use of screening for BCVI, the increasing use of whole- body mdCTA contrast imaging with a single spiral acquisition and a single contrast injection means that less expenditure may be required for dedicated screening for BCVI. Screening for BCVI may be cost-effective even when DSA is used to screen for BCVI. In the study by Cothren et al., 187 patients with asymptomatic BCVI were detected, preventing 32 strokes in the authors’ estimation. The authors concluded that at $6500 per DSA, the expense per stroke avoided was about $154,000 and that screening with DSA was therefore cost-effective [11].
The Clinical Scenario
A 42 year old woman is admitted with multi-system blunt trauma and is neurologically intact. Fractures involving the transverse foramina of the cervical vertebrae- a recognized radiographic risk factor for blunt cerebrovascular injury- are discovered on screening CT of the Cervical Spine, but angiographic screening for BCVI not performed, and antithrombotic/ antiplatelet therapy not initiated. 24 hours following admission the patient develops a large ischemic stroke in the territory of the left middle cerebral artery. Subsequent angiographic imaging with mdCTA reveals Grade 2 BCVI of both carotid arteries. The patient demonstrates significant disability at follow up.
CONCLUSION
The use of angiographic imaging to detect cerebrovascular injury based on appropriate clinical and imaging screening criteria in patients with blunt trauma may be an under-recognized opportunity to reduce morbidity and long-term disability in these patients. Injuries when discovered should be treated appropriately and follow up imaging performed to determine the evolution of the injury.
CONFLICTS AND DISCLOSURES
None of the authors have any conflicts to declare or disclosures to make.
FUNDING
None declared.


