All published articles of this journal are available on ScienceDirect.
Dynamic Analysis of Vibration, Muscle Firing, and Force as a Novel Model for Non-Invasive Assessment of Joint Disruption in the knee: A Multiple Case Report
Abstract
We present a new method for understanding knee pathology through non-invasive techniques. The combination of electromyography (EMG), vibroarthrographic (VAG), and force analysis in proposed to examine the force transfer between unhealthy and healthy knees. A multiple case report is presented to demonstrate the technique and its potential application for future study. The comparison of four individuals’ knee characteristics will be explained using this innovative methodology.
1. INTRODUCTION
Existing methods for the detection of changes in knee joint pathology as determined by the orthopedic and podiatry community often include invasive techniques such as arthroscopy [1-3] as well as noninvasive assessment such as Computed Tomography (CT), Magnetic Resonance Imaging (MRI) [4, 5], the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) subjective knee pain ratings scale [6-13], and X-ray imaging [4, 5]. To date, arthroscopic analysis has been the standard assessment and is considered the most reliable assessment of knee pathology. However, as Kim et al [14, 15] observed, image-based techniques provided anatomical images of the joint cartilage, but failed to characterize the functional integrity of the cartilage in terms of its softening, stiffness, or fissuring [3-5].
The other limitation of these image-based techniques includes diagnoses made based on still images. Knees are, perhaps, the most dynamic joints of human bodies, and pain and other shear force traumas are associated with unplanned cutting and deceleration activities at high speeds [16-21]. This observation has led to the exploration for technologies that capture changes in soft tissue like the meniscus, allow the examiner to assess the knee joint functionality dynamically, as well as track and monitor continued degeneration in a non-invasive way [22-28]. This situation has motivated us to develop a methodology of dynamic analysis of knee functionality.
Dynamic analysis has additional advantages. It enables us to analyze the data based on physics. Physics-based analysis provides information that statistics-based analysis does not. Each test subject has unique features such as muscular strength, bone formation and history of injury etc. [29-33]. Therefore, for accurate assessment, statistical data requires a large sampling size from a wide variety of groups. Dynamic analysis affords the inclusion of acute features and dynamic changes of individuals in situ [34-36]. In addition, it should be noted that nature of each group can change over time. For instance, with the increase of life expectancy, age groups must be redefined periodically. Additionally, lifestyle changes rapidly as well [37-48]. With on-line shopping being more popular, people tend to walk much less in their daily life. This is evidenced by frequent redefinition of hypertension [49]. On the other hand, physical laws are universal and mostly permanent. It is our idea to interpret acquired data as much as possible based on Newtonian dynamics so that analysis is applicable to all test subjects in common.
Along with dynamicity, systematic analysis based on multiple types of data is of extreme importance. Recent techniques for signal analysis have included time frequency analysis, wavelet decomposition/pursuit decomposition, dynamic weighted fusion classifiers [50], auto regression equations [51], least square analysis of frequency data, linear prediction, time warping, and modeling [51], techniques to examine variance and entropy within a signal. While these classifications have been useful, the complexity of knee pathology is often seen in muscular areas such as the quadriceps, biceps quadriceps femoris, semi-tendinosis, gastrocnemius, and tibialis [50-57]. Additionally, when movement patterns become inefficient, greater force is exerted to perform sitting to standing tasks [58], and greater compensations that acoustical sensing alone cannot describe. Relative to the sports medicine profession, the translation of force through the lower body kinetic chain can result in altered features of crepitus, muscle firing synchrony and amplitude, and gait changes [59, 60]. Through the process of measuring multiple sources of force translations, it may be possible to develop a transfer function that more holistically describes the pathology of the knee joint and can be built to classify subtle differences in pathology of movement [60, 61].
The purpose of this report is to provide a summary of our past four years of research in which we conducted a multiple case analysis employing the framework of force-plate and VAG (Vibroarthrographic) acoustic signals of the knee joint relating to normal and affected knee movements. It is done by tracking changes in quadriceps, hamstring, and tibialis muscles with Electromyography (EMG) signals. Also, EMG to force transfer functions were compared to determine if consistent changes occur between healthy and unhealthy knees [62-65]. Thus, the investigation was a description and confirmation of knee conditions using multiple measurements across four individuals with pathology or abnormality in the knee capsule. Moreover, the research team attempted to answer the following: Do variances in the acoustic signals, EMG amplitudes, and force output signals identify reliable changes across affected and unaffected knees during movements in sitting to standing (squat movements) and marching movements at varying rates?
2. BACKGROUND
The exploration of novel assessments of knee pathology stem from the cost and limitations of current assessment such as MRI. While MRI assessment has been a clinically useful, a review by Yusef et al. [61] examining data from 22 studies of knee pain and osteoarthritis as assessed through MRI has concluded that the level of evidence from MRI related to knee pain and Osteoarthritis (OA) were limited and conflicting. So precision of MRI to detect, predict, and differentiate tissue damage is, at best, still evolving.
The cost of MRI for the knee with contrasts, according to Healthcare Bluebook on 4/21/18, estimates rough $1071.00 as a fair price for this assessment. While the MRI also gives a good depiction of tissue densities and images that can be quantified, it is not dynamic and may not account for movement related changes in tissue for a more comprehensive diagnosis. It is also likely that VAG would be similar to the cost of X-ray which hover around $84.00 per assessment [64, 65].
When a tissue is swollen, the MRI images are often less clear, so timing of the assessment relative to the injury can often impact the accuracy. VAG, at present, does not differentiate tissue damage, but through regression models, and further testing, it can be improved. This technology has been evolving since the early 1980’s but with greater sensor capabilities, improved software, multi-model integrating of EMG, force plate translation, and wireless/portable capabilities, this assessment could benefit knee assessment in multiple settings. Moreover, VAG could serve as a cost effective, dynamic assessment that reflects real-time changes within the knee, even with synovial distortion.
VAG techniques detect changes in the knee joint at the internal and external articular surfaces through knee sounds. The knee acoustics were measured first through stethoscope and then vibratory sensors [62]. VAG allows for the assessment of useful indicators of the popping (crepitus) sound, gliding of the patella-femoral joint, and the potential multiple frequencies emitted when the movement of the articular cartilage surface is studied [62, 63]. Moreover, these sounds have been linked to changes in Osteoarthritis (OA) of the knee as graded by Kellegren and Lawrence scores (KL) in the patellofemoral joint. The KL grading scale is for classifying the status of OA into five grades, with 0 representing normal and 4 the most severe disease: Grade 0: None (no OA), Grade 1: Doubtful (no narrowing of joint space and possible osteophyte), Grade 2: Minimal (definite osteophytes and possible narrowing of joint space), Grade 3: Moderate (moderate osteophytes, definite narrowing of joint space, some sclerosis and possible deformity of bone contour), Grade 4: Severe (large osteophytes, marked narrowing of joint space, severe sclerosis and definite deformity of bone contour). One recent study using VAG demonstrated moderate to high correlations with KL classifications (r ranging from 0.53-0.56) and sensitivity to predict OA and correct prediction of OA classification analysis to be as high as 89.52% and 82.8%, respectively [8-13]. In this manner, VAG signals could help avoid unnecessary exploratory surgery because they demonstrate good discriminant validity and sensitivity for differing levels of degeneration. Additionally, VAG could enhance assessment of appropriate candidates for surgery or knee replacement while also tracking for joint degeneration without surgery or expensive imaging techniques. Thus, assessment could be performed in a cost-effective, noninvasive manner. Perhaps the most logical reason for the lack of development in this area has been the complexity of interpreting the wavelet data in the time and frequency domains, and signal processing techniques to arrive at an accurate estimation of normal versus abnormal patterns.
3. MULTIPLE-CASE REPORT
3.1. Experimental Arrangement and Procedures
After formal approval of Institutional Review Board for Human Subjects was granted, volunteers were recruited to perform squatting and marching tasks for the experiment. The criteria for inclusion were: 1) previous history of knee injury, 2) no current contraindications that squatting or marching would be detrimental to the knee, and 3) a health history that does not preclude any normal exercise.
Four subjects were selected and tested from a variety of age groups. Subject 1 was an 18-year-old male with no specific knee issue. Subject 2 was a 69-year-old male with severe arthritis in both knees. Subject 3 was a 25-year-old female with a torn meniscus of her right knee. Subject 4 was a 68-year-old male who complained of chronic pain in both knees (under exercises) but recent MRI or other medical examination did not indicate any issue [33].
To examine force, a bertec force plate (Columbus, OH) measured force and torque transfer across 6 degrees of freedom (Tsuneo Yoshikawa, Foundation of Robotics: Analysis and Control, The MIT Press, Cambridge MA 1990) (force parallel to the x, y, and z axes, torque around the same three axes) during sit-to-stand movements and marching movements. These recordings were integrated into an electrophysiogram (BIOPAC MP 150, Goleta, CA) so that acoustical (vibrational) and force sensations were recorded simultaneously. All were sampled at 1000 samples per second and were saved as Excel files for Matlab analyses later. A 10-lead Delsys EMG system (Natick, MA) recorded surface muscle activity, sampling at 1,000 samples per second. Thus, the data set consisted of ground force and torque measured with a force plate, knee vibration measured with a vibration sensor attached to the inner side of the knee, and EMG signal measured with a set of EMG sensors. The xyz-coordinate axes are affixed to the force plate and the locations of the sensors are shown in (Fig. 1).
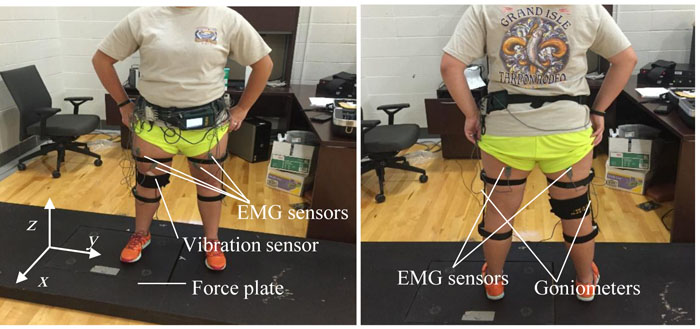
The test subjects performed two types of activities; marching and squatting, with one foot on the force plate while the other foot in on an elevated surface to align the stance. The force plate and vibration sensor signals were acquired by a Biopac TM package, and the EMG signals were acquired with a Delsys TM package. The acoustic sensor was attached to the subject’s knee between the medial condyle of the tibial bone and the medial condyle of the femur. An electrode gel was used on the surface of the sensor. Once the sensor was in place, it was secured with a medical tape wrapped around the knee. The vibration sensor was attached to the leg that was placed on the force plate. After testing the first knee, the vibration sensor was switched over to the other leg that was placed on the force plate for the second round of the test. The force plate measured the x-, y- and z-components of the force and torque vectors. Ten EMG sensors (five per leg) and two goniometers (one per leg) were attached to the subject. The goniometer was used to assure that the subjects bent their knees properly. The five EMG sensors were attached to the belly of the vastus lateralis, vastus medialis, rectus femoris, biceps femoris, and the anterior tibialis muscle. Once all EMG sensors were in place, the goniometer was attached, lined up with the greater trochanter of the femur bone and the lateral malleolus of the fibula [33]. The subjects then performed a series of marching and squatting at set frequencies guided by a metronome for 70 seconds. Here frequency was defined as the completion of exercise, e.g., 2 Hz marching was defined as two cycles of marching that caused the foot on the force plate land on it twice in 1 s. In other words, the subject touched the ground with the two feet four times in total every second, or 240 times in one minute. The subject was guided by 240 beats per minute on the metronome for 2 Hz marching. Similarly, 1 Hz squatting meant one crouching and standing motion in 1 s. The subject was guided by 120 beats on the metronome to crouch and stand.
The signal from each sensor was sent to a channel of the Biopac TM or Delsys TM system. The two systems were synchronized manually. All the signals were analyzed in the frequency domain via Fourier transform of the time series collected by the Biopac and Delsys systems at the sampling rate of 1 kHz (1000 samples per second).
3.2. Marching Activity
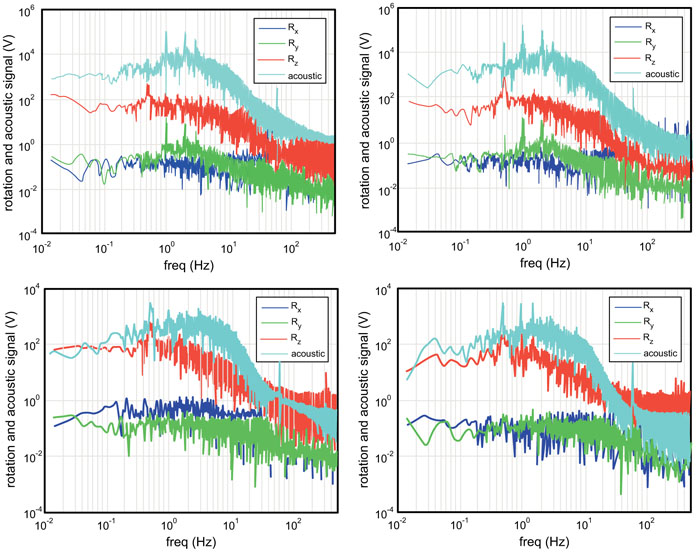
Fig. (2) shows subject 1’s (upper) and subjects 2’s (lower) results from the vibration sensor and torque around the three coordinate axes while performing the marching activity at 0.5 Hz. The left graphs are signals from the left knees and the right graphs are signals from the right knees. From these signals, a number of features that characterize each knee can be drawn.
The vibration sensor (acoustic) signal in Subject 2 is much weaker (approximately by two orders of magnitude weaker at the peak value) than in Subject 1. This can be attributed to the presence of fluid in the knee cavity. Subject 1’s vibration signal has a peak at 1 Hz (the double of the frequency of activity), while Subject 2’s vibration signal barely shows a peak at 1 Hz. The 1 Hz peak indicates that the vibration sensor detects a signal when the foot is raised from the force plate and then lowered back down on the force plate. It is further argued that the vibration signal is associated with the knee’s flexion-and-extension motion, which is correlated with Ry signal. The fact that Subject 2 does not show the 1 Hz peak indicates that the vibration signal is weaker when the foot is raised or lowered [13]. This observation is consistent with the above speculation that Subject 2 has fluid in his knee cavity. It is also consistent with the fact that Subject 2’ Ry signals is an order of magnitude lower than Subject 1.
Another interesting feature observed in the vibration signals from the two subjects is their spectral shapes around the frequency of activity. In oscillation dynamics, spectral peak broadening represents energy damping [62] which normally results in attenuation of the signal at the frequency of activity, leading to reduction in the peak value. This is perfectly consistent with the above observations that Subject 2 exhibits a two orders of magnitude lower peak value in the vibration sensor signal and an order of magnitude lower peak in Ry signal than Subject 1. It is likely that the fluid in the knee cavity reduces the vibration associated with the flexion-and-extension motion. Also notice that Subject 2’s left knee exhibit significantly broader peak at 0.5 Hz than his right knee, indicating that his left knee is more problematic than the right.
Now we look at torque signals. As will be discussed in the following paragraphs, relative strengths of Rx, Ry, and Rz indicate stability/instability of knees. Both subjects demonstrated strongest torque around the z axis with the highest spectral peak at 0.5 Hz. With the activity frequency of 0.5 Hz and the experimental arrangement where one foot lands on the force plate at a time, the force plate detects the signals every two 2 s or 0.5 Hz in frequency. The appearance of the spectral peak at 0.5 Hz is accountable. The fact that the Rz signal is stronger than Rx and Ry indicates that when the foot lands on the force plate, the subject adjusts internal-external rotation by exerting the torque Rz [62].
In marching motion, the torque around a vertical axis indicates instability because the internal-external rotational motion (i.e., to move the foot as if squishing a bug) is not the dominant mode of motion [14, 15]. Similarly, rotational motion associated with Rx (outside-inside tilting motion of a foot) is not the dominant mode. Instead, the flexion-and-extension should be the dominant mode, since the subject kicks the ground (force plate) backward. This motion should be exhibited by the Ry signal at the frequency of the activity. Hence, it can be said that a strong signal in Ry is an indicator of a healthy knee, and a strong signal in Rx or Rz is an indicator of weakness.
Subject 2 shows a factor of three-to-five lower signal in Ry (the healthy knee indicator), and two-orders of magnitude higher Rz (the weak knee indicator) than Subject 1. In addition, Subject 2’s Rz spectrum is much broader than Subject 1’s around the frequency of activity. As mentioned above, spectral peak broadening represents higher energy damping due to loss mechanisms. In the case of Rz, it is possible that friction between the foot and the force plate surface due to unstable (less controlled) motion of the foot is the main loss mechanism. These tendencies are greater in Subject 2’s left knee, which is the more problematic one as indicated by the vibration signal. Rx signals also support this observation. Subject 2’s Rx signal is higher than his right knee, and much higher than Rx of Subject 1. All these observations are consistent with each other, leading to conclusions that Subject 2 has weaker knee than Subject 1, and Subject 2’s left knee is weaker than the right.
3.3. Squatting Activity
Fig. (3) shows the Fourier Spectra [67] of the Subjects 3’s left and right knees during the 1 Hz squat activity. This subject has a torn meniscus in her right knee. In both knees, a peak appears at the activity frequency of 1 Hz. The three torque signals have approximately the same level of magnitude except that the right knee’s Ry is somewhat lower towards the low frequency end. The same level of magnitude in the three torque signals indicates in the squatting motion the foot exerts torque equally in the three rotational degrees of freedom [68]. The lower Ry seen in the right knee indicates that the torn meniscus weakens the torque for the flexion-and-extension. The right knee shows much broader peak in all signals, clearly indicating instability due to the torn meniscus.
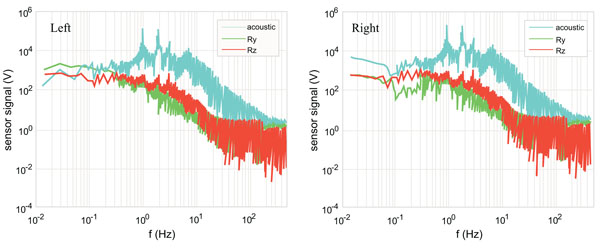
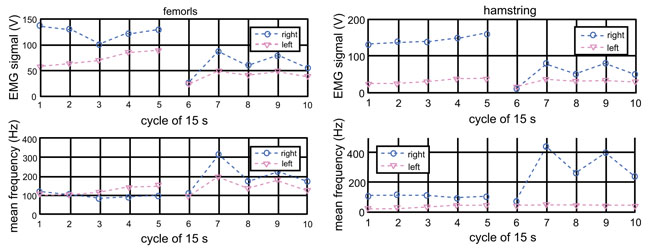
Fig. (4) plots the EMG signals from the quadriceps femoris and hamstring (biceps femoris) of Subject 3. The EMG measurements were made for 70 s, and Fourier transform was computed for five of 15 s (short Fourier transform) of the total duration and the set of measurements was repeated twice. The horizontal axis of Fig. (4) indicates the cycle of the 15 s data segments. There was an approximately 5-minute break between the first and second sets. The top graph of Fig. 4 is the magnitude of the EMG signal’s Fourier spectrum and the bottom is the mean frequency for each 15 s segment. The left graph is for the quadriceps femoris and the right graph is for the biceps femoris or one of the hamstring muscles. When a muscle is fatigued, the EMG-signal is known to increase in the amplitude and decrease in the mean frequency. Fig. (4) does not show clear tendency of fatigue in either leg or muscle. In fact, the mean frequency appears higher in the second set than the first. It is possible that the frequency is lower in the first set because the muscles are not warmed up. It is clear that the right quadriceps femoris and hamstring exhibit higher muscle amplitude than the left. This indicates that the right leg muscles are working harder (less efficiently, as will be discussed shortly) because of the meniscus issue.
Since the EMG signal is an indicator of muscle’s energy and the torque around the y-axis (the torque for the flexion-and-extension) is important for the squatting activity, the ratio of the torque to the EMG (Ry/EMG magnitude) can be used to evaluate the efficiency of the muscles for the activity. Fig. (5) plots the ratio (called the EMG to Ry transfer function) for the quadriceps (left graph) and hamstring (right graph).
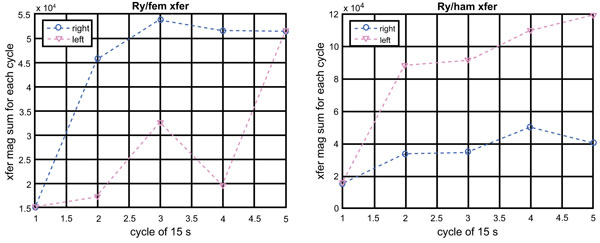
Contrary to our prediction, the right femoris shows higher efficiency than the left femoris. On the other hand, the left hamstring shows higher efficiency than the right hamstring. We suspect that as efficiency of movement wanes, subjects become quadriceps dominant and the protective effect of balanced hamstring-to-quadriceps ratios decline. It is only speculative at this point: we do not have a more convincing explanation for this observation at this time.
Figs. (6-8) are the same type of plots as Figs. (3-5) for Subject 4 (68-year-old male complaining of knee pain under exercise with no medical evidence of disorder). The Fourier spectra of the vibration and torque signals Fig. (6) are very similar between the left and right. The peaks at the frequency of activity are, in fact, very sharp. Unlike the case of Subject 3, the EMG signals’ magnitude and frequency [62] appear similar between the left and right Fig. (7). The efficiency also appears the same between the left and right . These observations indicate that both knees of Subject 3 behave similarly without indication of loss mechanism such as the presence of knee cavity fluid and frictional nature of the knee/foot motion, which would cause spectral broadening.
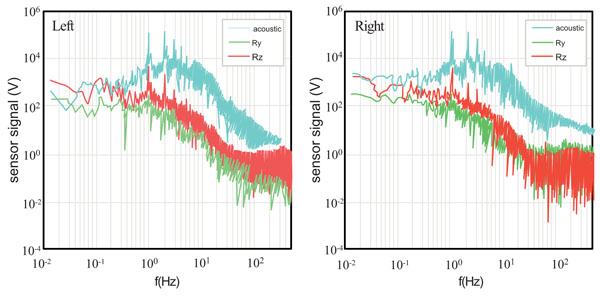

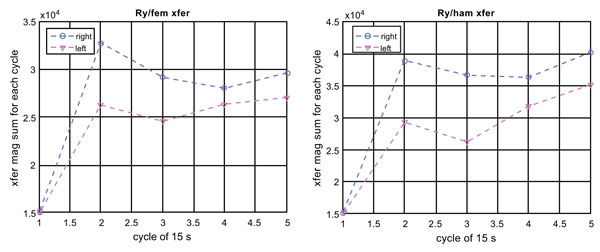
Fig. (8) plots quadriceps EMG to Ry transfer functions of Subject 4 (with no identified knee disorder), which corresponds to Fig. (5) for Subject 3 (right torn meniscus). Comparison of Figs. (5 and 8) indicates that Subject 4, although he complains discomfort while doing exercises, has the same efficiency between the two knees. This may be why the X-ray image does not indicate any structural defects. This is different to Fig. (5) for Subject 3 who has a torn meniscus on the right knee.
DISCUSSION AND CONCLUSION
Human knee functionality, when examined by acoustical sensor, muscular firing, and force, can produce data that describe knee behavior related to torque exerted around an axis. Moreover, these measures produced some important trends that were gathered with a non-invasive, cost-inclusive technology that has tremendous potential in the sport and exercise medicine setting [69-75]. The signals were analyzed in the frequency domain. For each subject, the data were discussed in association with the knee condition specific to the subject. Transfer functions [76-83] became increasingly useful as the multilayer approach to knee sounds, ground force actions, and muscle firing, where they were compared in this complex manner. Key observations made during the multiple case report included;
- Degrees of freedom around the Rz axis demonstrated clear differences from internal-external rotation between an injured and uninjured knee for subjects 1 and 2.
- Torque around the Ry axis during marching movements demonstrated unique changes in flexion-extension movements between injured and uninjured knees, sound dampening in the knee of subject 2 indicated increase in synovial fluid and lower peak magnitude values as causes of lower vibration in this frequency range.
- Subjects 1 and 2 had differing orders of magnitude in the Rz and Ry axis indicating compensation of internal and external rotation through increased changes in flexion-extension characteristics.
- Rx was similar in both subjects 1 and 2 indicating calibration was accurate.
To truly support these findings, a larger scale study is underway to develop more accurate and complex screening tools for knee pathology. The aim of the next study will be to reproduce these results and provide validation for prediction of knee pathology non-invasively. The present multiple case presentation demonstrates a multi-layered analysis of knee sounds, muscle firing, and force generation that can be replicated and improved to aid sport and exercise medicine professionals assess knee pathology.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study was approved by the Institutional Review Board (IRB).
HUMAN AND ANIMAL RIGHTS
No Animals were used in this research. All human research procedures followed were in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013.
CONSENT FOR PUBLICATION
Informed consent was taken from the patients when they were enrolled.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
We would like to acknowledge Jessie Hatchett for his contribution as am electronics consultant and service provider throughout the project.


