All published articles of this journal are available on ScienceDirect.
Premotor Gray Matter Volume is Associated with Clinical Findings in Idiopathic and Genetically Determined Parkinson’s Disease
Abstract
In the present voxel-based morphometric study, we investigated whether the severity and duration of disease are associated with alterations in gray matter volume (GMV) in symptomatic Parkin mutation carriers (sPARKIN-MC) and patients with idiopathic Parkinson’s disease (iPD). Regression analyses revealed different negative correlations between GMV in cortical motor areas and the severity as well as the disease duration in sPARKIN-MC and iPD patients. SPARKIN-MC showed a less involvement of cortical motor areas, in particular in the supplementary motor area (SMA) than iPD patients. Specifically, in iPD patients, but not in sPARKIN-MC, there was a negative correlation between the SMA degeneration and the UPDRS-II item freezing. The different degeneration patterns may mirror diverse kinetics of the disease progress in these two groups of PD patients with different underlying etiologies.
INTRODUCTION
Although the origin of the disease currently remains unknown in the majority of patients with idiopathic Parkinson’s disease (iPD), about 5% of all cases are associated with a mutation in a single gene. A monogenic cause is much more frequent among patients with early-onset parkinsonism (10-20%), of which mutations in the Parkin gene are the most common known cause [1]. Although there is substantial clinical overlap, Parkin-associated PD tends to manifest earlier, has a milder course and a better response to levodopa than iPD patients [2].
While some previous MR-based morphometric studies failed to show regional differences in cortical volume between iPD patients and controls [3], other morphometric studies found a reduction of gray matter volume (GMV) in frontal [3, 4], temporal [3-5] and parietal areas [4], hippocampus [5] and anterior cingulate cortex [5]. Here, we used voxel-based morphometry (VBM) to test for a linear relationship between clinical features of PD and regional GMV. Since we made the clinical observation that the symptomatic Parkin mutation carriers (sPARKIN-MC) had less freezing problems than iPD patients, special emphasis was placed on the investigation of the pathoanatomical basis of this differential clinical manifestation.
METHODS
We compared structural MR-images of 9 sPARKIN-MC, identified in large-scale genetic studies [6], (two females, mean age: 52.3 ± 3.8 years) and 14 iPD patients (eight females, mean age: 50.9 ± 1.1 years) with 24 age- and sex-matched healthy controls (11 females, mean age: 52.1 ± 1.5 years). The motor section of the Unified Parkinson’s Disease Rating Scale (UPDRS-III) and a standardized clinical psychiatric examination including screening for dementia (Mini-Mental State Examination [MMSE] were performed. Details of demographic and clinical findings have been published elsewhere [7]. None of the participants suffered from dementia. All subjects and patients gave their informed written consent for participation in this study, which was approved by the local ethics committee in accordance with the ethical standards in the declaration of Helsinki.
T1-weighted FLASH-3D MR-images (echo time [TE] = 5ms; repetition time [TR] = 15ms; flip angle = 30°; voxel size 1x1x1 mm3) were assessed on a 1.5 T scanner (Symphony, Siemens, Erlangen, Germany). Morphometric analyses were performed using SPM2 software (FIL, London, www.fil.ion.ucl.ac.uk/spm) and the modified VBM protocol. Details of the VBM procedure are described elsewhere [8].
VBM employed a categorical comparison between patients and controls (ANOVA; GMV threshold: 0.25) as well as simple regression analyses with motor scores and disease duration as explanatory variables. We hypothesized that regional changes in GMV would occur primarily in the supplementary motor area (SMA), primary motor cortex (M1) and premotor cortex (PMC). Given our a-priori hypothesis, we applied a region-of-interest analysis for voxels within these primary and premotor areas using the WFU-PickAtlas (ANSIR, Wake Forest University) as anatomical reference. The statistical threshold was set at pFDR < 0.05 after correction for whole brain volume. For region-of-interest analysis, small volume correction (SVC) was applied using a spherical volume of 8 mm.
RESULTS
While mean UPDRS-III scores were matched between groups (sPARKIN-MC = 22.0 ± 4.3; iPD patients = 22.7 ± 3.3; t-test: p = 0.89), the item “freezing” in the UPDRS-II showed trend towards a difference: sPARKIN-MC reached a median of 0.50 (range: 0–1) and iPD patients of 1.00 (range: 0–3; Wilcoxon-test p = 0.06). There was no difference in mean disease duration, defined as time from symptom onset, between groups (sPARKIN-MC = 14 ± 3.1 years vs. iPD patients = 12.1 ± 1.0 years; t-test: p = 0.49).
Voxel-by-voxel comparison of cortical GMV revealed no significant differences between sPARKIN-MC, iPD patients, and age- and sex-matched healthy controls. Within-group regression analysis showed an inverse linear relationship between total UPDRS-III scores and regional GMV in left SMA, left M1, right ventral PMC and bilateral dorsal PMC in iPD patients (Table 1). In sPARKIN-MC only the dorsal PMC showed a bilateral decrease in GMV with increasing UPDRS-III scores. When correlating the morphometric data with the clinicial UPDRS-II item which scores “freezing”, regression analysis revealed a linear GMV decrease with freezing in the left SMA (as well as a trend in the right SMA) in the iPD group (Table 1, Fig. 1) but not in the sPARKIN-MC group. When using mean disease duration as explanatory variable, a linear decrease in GMV with disease duration was found in left dorsal PMC, right M1 and left SMA, again only in iPD patients (Table 1).
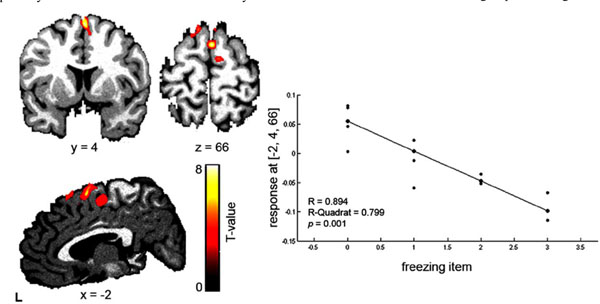
Clinical correlation between the UPDRS-II freezing item in iPD patients.
Coordinates and Gray Matter Values for Cortical Motor Areas in sPARKIN-MC and iPD Patients
| Region | Side | MNI Coordinates in mm | T-Value | Z-Score | pFDR (SVC) | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Regression analysis with UPDRSIII a) sPARKIN-MC |
|||||||
| SMA | - | - | - | - | - | - | - |
| dPMC | R | 22 | -7 | 60 | 7.76 | 4.04 | 0.009 |
| dPMC | L | -26 | -12 | 61 | 6.38 | 3.70 | 0.015 |
| b) iPD patients | |||||||
| SMA | L | -3 | 4 | 60 | 4.59 | 3.42 | 0.048 |
| M1 | L | -63 | -6 | 27 | 4.35 | 3.31 | 0.021 |
| vPMC | R | 64 | 8 | 19 | 5.57 | 3.84 | 0.014 |
| dPMC | R | 31 | 10 | 63 | 4.96 | 3.59 | 0.031 |
| dPMC | L | -63 | -5 | 28 | 4.86 | 3.55 | 0.019 |
| Regression analysis with freezing score | |||||||
| a) sPARKIN-MC | |||||||
| SMA | - | - | - | - | - | - | - |
| b) iPD patients | |||||||
| SMA | L | -2 | 4 | 66 | 6.92 | 4.31 | 0.002 |
| SMA | L | -6 | 24 | 61 | 5.06 | 3.63 | 0.023 |
| Regression analysis with disease duration a) sPARKIN-MC |
|||||||
| SMA | - | - | - | - | - | - | - |
| b) iPD patients | |||||||
| SMA | L | -18 | -8 | 65 | 3.95 | 3.10 | 0.046 |
| M1 | R | 24 | -28 | 63 | 4.92 | 3.58 | 0.031 |
| dPMC | L | -45 | -10 | 39 | 4.92 | 3.58 | 0.015 |
dPMC: dorsal premotor cortex, vPMC: ventral premotor cortex M1: primary motor cortex, SMA: supplementary motor area.
The clinical regression analysis with the freezing item, obtained from the UPDRS-II revealed a decrease in GMV in the SMA in iPD patients (none in sPARKIN-MC). On the right side the negative correlation plot of the regression analysis between the freezing item (UPDRS-II) and the decrease in grey matter density in the SMA (x = -2. y = 4, z = 66, Z = 4.31, pFDR = 0.002, R = 0.89) in iPD patients is displayed (for better visualisation a threshold p < 0.05 was used).
DISCUSSION
Using whole-brain VBM, we found a gradual reduction in GMV in cortical motor areas that shows a linear relation to the severity of individual motor symptoms and duration of disease in iPD patients. This linear relationship between motor symptoms and cortical VBM was less pronounced in the sPARKIN-MC group, especially in the SMA. This may represent an anatomical correlate of the clinical observation that sPARKIN-MC have slower progression of disease and show less freezing problems than iPD patients [6].
In sPARKIN-MC, a linear decrease in GMV with the severity of motor symptoms was only significant in the dorsal PMC, and there was no clear relationship between GMV in motor cortical areas and disease duration. The weaker relationship between cortical GMV and clinical features of PD might indicate that cortical motor structures are less affected by neurodegeneration in sPARKIN-MC than in iPD patients. A relative sparing of cortical motor areas might at least partially account for the slower and more symmetric onset of symptoms, the better response to L-Dopa and the generally favourable outcome in sPARKIN-MC [6].
Only patients with iPD displayed a linear reduction in GMV with the severity of motor symptoms in the rostral SMA. They also had more freezing problems than sPARKIN-MC, and showed a linear decrease in GMV in the SMA depending on the individual freezing score. These observations tie in with current concepts of the role of the SMA in motor dysfunction in iPD, especially freezing. The SMA belongs to the mesial initiation motor system, whereas the lateral premotor areas are part of the lateral integrative motor system [9]. A malfunction of the mesial motor areas in iPD patients have been reported in several electrophysiological and neuroimaging studies, and may constitute a functional correlate for the difficulties to initiate movements internally and to the clinically observed freezing problems in iPD patients. A decreased activity of the rostral SMA, anterior cingulate cortex, and lateral prefrontal cortex was found in iPD patients during the execution of free chosen hand [10] or complex finger movements [11] as demonstrated by PET and functional MRI. Accordingly, electroencephalographic (EEG) studies showed a relative „deactivation“ of the fronto-central cortex in the preparation phase of finger movements [12] and a shortened period of pre-movement desynchronisation in the alpha- and beta-frequency bands [13] in iPD patients. These results have been interpreted as evidence for a deficient activity of the SMA via the medial premotor basal ganglia loop in untreated iPD patients.
A correlation between the degeneration of the lateral PMC and the severity of motor symptoms was found in both groups of patients. The dorsal PMC is supposed to play a crucial role in the preparation of externally triggered movements (e.g. through sensory stimuli) [14], and therefore it seems to be predominantly active during the planning and execution of sensory guided movements [15]. Event related EEG studies showed a movement related increase in activity of the dorsal PMC in PD patients [12]. Also in PET and functional MRI studies, an increased activity of the dorsal PMC could be observed during sequential finger movements [16], complex finger movements [11] and free choice finger movements. The increased activity in the dorsal PMC has been interpreted as a sign for a compensatory reorganization of the motor system with stronger use of the cerebello-parieto-premotor loops as a reaction to the deficient function of the medial PMC and the basal ganglia loop [16]. The results of the present morphometric study raise the possibility that the capacity to compensate in this lateral premotor loop may fade during the course of disease with increasing severity of symptoms. This hypothesis remains to be addressed in future studies.
CONCLUSIONS
The present results show that MRI morphometry in conjunction with genetic and clinical characterization of patients provides a powerful tool to achieve a better understanding of the morphometric correlates and regional function-structure relationships in genetically determined PD and iPD.
ACKNOWLEDGEMENTS
The study was supported by grants of the Deutsche Forschungsgemeinschaft (Kl-1134-2-2, Kl-1134-3-1, RE 2841/1-1), the Parkinson’s Disease Foundation and the South Tyrolean Parkinson Association. CB (01GO0510), HS (01GO0511), and FB (01GO05102) were supported by a structural grant of the Bundesministerium für Bildung und Forschung (BMBF) to NeuroImageNord. CK, HS and FB were further sponsored by the EU-FP6-Grant (GENPARK; EU-LSHB-CT-2006-037544). Position of CK was supported by the Volkswagen Foundation, and KR (E06-2008) and CK by intramural grants from the Medical Faculty, University of Luebeck.


