All published articles of this journal are available on ScienceDirect.
Primary Intracranial Choriocarcinoma Located in the Suprasellar Region
Abstract
A 10 year old girl was admitted to our hospital due to headache, nausea, and weight loss for about half a year. She also had visual field disorders. Suprasellar tumor was found by X-ray computed tomography, and magnetic resonance imaging showed a ring-like lobulated enhanced mass with hemorrhage and necrosis. Biopsy of this lesion showed primary intracranial choriocarcinoma on histopathological examination. The serum human chorionic gonadotropin (hCG) level was measured after the biopsy and was elevated at 71,298.2 IU/L. The patient died due to hydrocephalus caused by an increase in the size of the tumor with a larger amount of hemorrhage than the preoperative features. If young patients present with a suprasellar lobulated mass with hemorrhage, the serum hCG level should be measured before operation.
INTRODUCTION
Primary intracranial choriocarcinomas (PICCCs) are one of the rarest and most malignant primary intracranial germ cell tumors (GCTs), accounting for 3%-5% of primary intracranial GCTs [1]. PICCCs are usually located in the pineal and suprasellar regions of the brain and must be differentiated from other tumors occurring more commonly at these sites because treatment and prognosis would be different [2]. A correct diagnosis is very important for determining the proper therapeutic strategy for PICCCs. Neuroimaging evaluation and serum human chorionic gonadotropin (hCG) level determination are useful in distinguishing PICCCs from other lesions. Here we report the case of a patient with PICCC to emphasize the importance of early and accurate diagnosis before operation.
Case Report
A 10 year old girl was admitted to our hospital with visual field disorders. The patient presented with progressing headache, nausea, and weight loss for approximately half a year. The past history and family history of the patient were unremarkable. She was initially diagnosed as having gastroenteritis on admission; however, abducens nerve palsy on the left eye and visual field disorders developed after the preliminary diagnosis. Non-contrast computed tomography (CT) of the head found a solitary mass with heterogeneous high density in the suprasellar region (Fig. 1a). Magnetic resonance imaging (MRI) showed a lobulated mass with a well-defined margin with mixed high-intensity and isointense signal on T1-weighted imaging (Fig. 1b) and mixed low-intensity and high-intensity signal on T2-weighted imaging (Fig. 1c). These imaging findings suggested the presence of large hemorrhage, necrosis, and small cyst in the tumor. The tumor showed a heterogeneous high-intensity area on diffusion-weighted images (Fig. 1d), low-intensity area on ADC map. (Fig. 1e). On contrast-enhanced MRI, the tumor exhibited a ring-like enhancement (Fig. 1f). Blood examination findings, excluding serum hCG level were all normal. The serum hCG wasn’t initially measured.

Brain non-contrast CT shows a heterogeneous high-density mass in the suprasellar region (a). On MRI, T1-weighted coronal images show a lobulated mass with mixed high-intensity signal in the suprasellar region (b). T2-weighted coronal slice image shows a lobulated mass with mixed high-intensity and low-intensity signal in the suprasellar region (c). Diffusion-weighted image shows a heterogeneous high-intensity mass (d), and ADC map shows low-intensity mass. (e). Coronal TI-weighted gadolinium-enhanced MRI shows a mass with ring-like enhancement (f).
Endoscopic tumorectomy was performed via the sphenoid sinus approach fourteen days after the initial plain CT. The tumor appeared to be hard. As total removal of the tumor was difficult, we only performed biopsy. Microscopic examination revealed that the resected tumor tissue was composed of mononucleated cytotrophoblastic cells (Fig. 2a) and large multinucleated syncytiotrophoblastic cells (Fig. 2b). No other germ cell components were present. Hemorrhage and necrosis were also confirmed (Fig. 2c). Immunohistochemical staining for hCG was strongly positive (Fig. 2d). Although there were no abnormal findings in the genital system based on physical and blood examinations at this time, these pathological findings were indicative of choriocarcinoma. The final pathological diagnosis confirmed the mass to be PICCC. Thereafter, the serum level of hCG was measured and was noted to be up to 71298.2 IU/L. A follow-up head CT scan twenty days after the biopsy showed a marked hydrocephalus and an increase in the size of the tumor with a larger amount of hemorrhage than the preoperative features (Fig. 3). It wasn’t confirmed that the tumor enlarging caused by just enlarging tumor that bled or the bleeding for biopsy because intratumoral hemorrhage had been
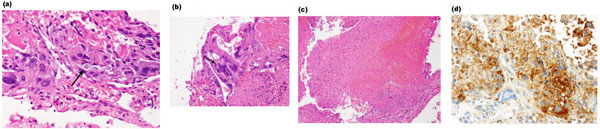
Photomicrographs of the biopsy specimens show mononuclear cytotrophoblast (arrow, a), a large multinuclear syncytiotrophoblast (arrow, b) with hemorrhage and necrosis (c). Immunohistochemical staining for hCG is strongly positive (d).
seen before the biopsy. Emergency surgery was performed for external ventricular drainage. A whole spine MRI scan showed that there was no metastasis in the spinal cord. Chemotherapy and whole brain and spinal radiation were initiated for the patient. Unfortunately, the patient died on the second day after initial chemotherapy.
DISCUSSION
PICCC, which is characterized by extra-embryonic differentiation along trophoblastic lines with histological identification of both cytotrophoblastic elements and syncytiotrophoblastic giant cells (STGC), accounts for majority of primary intracranial GCTs with high levels of hCG and is the most malignant primary intracranial GCT [1, 3]. PICCC usually arises in the second decade with a peak incidence at the age of 10-12 years and has a male predominance [1]. Regarding PICCC location, males show an increased incidence in the pineal region, the suprasellar region is more predominant in females. The clinical presentations of PICCC depend on the location and size and frequently include signs of increased intracranial pressure, visual changes, and endocrine abnormalities. Patients with PICCC in the pineal region frequently present with obstructive hydrocephalus, whereas those with PICCC in the suprasellar region usually present with visual field disorders. Precocious puberty, which results from the production of hCG, is one of some findings of PICCC in males, especially in patients younger than 12 years. However, in our case, the serum level of hCG was not measured before surgery because choriocarcinomas were not included in our differential diagnoses.
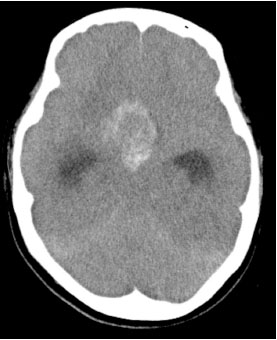
Post-operative axial slice head CT scan twenty days after the biopsy shows a marked hydrocephalus and a larger amount of hemorrhage than the preoperative features.
PICCC has some characteristic imaging features. The lesions are ovoid, round, or lobulated masses with well-defined margins and are most commonly located in the pineal and suprasellar regions [2]. PICCC is frequently hemorrhagic because trophoblastic tumors are often perfused by fragile vessels and due to the innate capacity of trophoblastic cells to invade and erode vessel walls [4]. On non-contrast CT scan, PICCC frequently presents as a heterogeneous high-density lesion. On T1-weighted images, the tumor may either be isointense or a mixture of high-intensity and isointense signal; in the latter case, high-intensity signal may be because of hemorrhage. In contrast, lesions are markedly heterogeneous with both low-intensity and high-intensity areas on T2-weighted images. Contrast-enhanced MR images may demonstrate marked heterogeneous enhancement or ring-like enhancement. Cystic or necrotic areas can also be seen in the tumors. Most tumors reported have mild to moderate peritumoral edema. Our imaging findings are similar to these previously reported findings [5].
PICCCs must be differentiated from other tumors occurring in the pineal and suprasellar regions because of different treatment strategies [6]. The differential diagnoses for PICCCs in the suprasellar include pituitary adenomas with apoplexy, craniopharyngiomas, and other GCTs (germinomas, teratomas, embryonal carcinomas, and yolk sac tumors). The differential diagnoses in pineal region include GCTs and pineocytomas. Pituitary adenomas with apoplexy are difficult to differentiate from PICCC by MRI, but serum hCG level would be useful. Craniopharyngiomas often show cystic and calcified components. On MRI, intratumoral hemorrhages are rare in the other GCTs. Germinomas demonstrate homogeneous enhancement. Teratomas show fatty and calcified components. Embryonal carcinomas and yolk sac tumors tend to be larger than PICCCs. Pineocytomas show homogeneous enhancement. Therefore, typical MRI features, combined with the patients’ age and serum hCG/β-hCG, may be useful in distinguishing PICCC from other lesions.
Positive staining for β-hCG is characteristic of choriocarcinomas [7]. Also, serum hCG can be a useful biological tumor marker for differentiating choriocarcinoma and GCTs. Markedly elevated serum hCG levels are found in choriocarcinomas, whereas mild elevations can be seen with other GCTs with STGC. Patients with pure choriocarcinomas or mixed GCTs with choriocarcinoma elements have levels of serum hCG more than 2000 IU/L, whereas the serum hCG levels in GCT patients without choriocarcinoma elements are less than 770 IU/L [6]. Serum hCG levels can also be used to accurately monitor response to treatment [8].
There is no established treatment for PICCCs because they are very rare, fatal, and highly resistant to standard treatment [2, 3]. Reports on several cases that are successfully treated suggest that a combination of surgery, chemotherapy, and radiation therapy may be effective and helpful [1-3, 8, 9]. Some investigators recommend surgery as first-line therapy for tumors that are small enough to be completely resected [1, 2]. Initial biopsy may lead to fatal tumoral hemorrhage and should be avoided [10]. The poor clinical outcome of PICCC may be ascribed to its high risk for fatal hemorrhage and propensity for extraneural/cerebrospinal fluid dissemination. Previous studies report that the major cause of early death is tumoral hemorrhage due to biopsy or radiotherapy [11, 12]. In our case, the patient dies from hydrocephalus, which is caused by the enlarging tumor with increasing intratumoral hemorrhage. Our experience suggests that the prognosis of these patients is improved to some extent if an early and appropriate diagnosis is made and if initial treatment focuses on the prevention of tumoral hemorrhage. Correct pre-treatment diagnosis would be very important for determining a therapeutic plan.
CONCLUSION
In conclusion, PICCC has characteristic MRI features, which are ovoid or lobulated shape and the presence of a large hemorrhage in the suprasellar or pineal region. If a young patient presents with a tumor and imaging features similar to those described above, PICCC should be considered and the serum hCG level should be measured before operation to confirm the diagnosis. Early and correct diagnosis of PICCC would be essential for appropriate treatment strategy.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
Declared none.


